
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ard
ary
vices
ct Zoom
▼ Part A
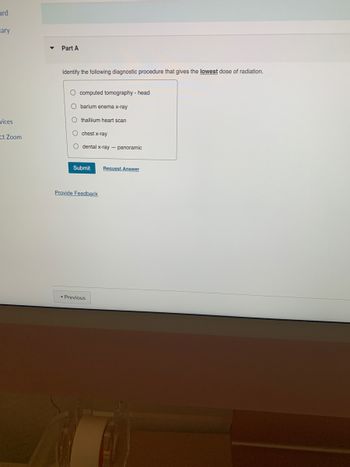
Identify the following diagnostic procedure that gives the lowest dose of radiation.
O computed tomography - head
O barium enema x-ray
Othalliium heart scan
O chest x-ray
O dental x-ray - panoramic
Submit
Provide Feedback
◄ Previous
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the binding energy in lithium-7. (1 amu = 1.6606 x 10-27 kg; speed of light = 3 x 108m/sec; 1 Joule = 1kg-m/sec2) A 7.05 x 10-27 J/atom B 6.3 x 10-12 J/atom C 4.21 x 10-27 J/atom D 3.99 x 10-12 J/atomarrow_forwardDO 14. O 16. QUESTION 15 The nuclear reaction shown below is an example of what type of process? Th→ Rn + He translation fusion Ofission alpha emission beta emission QUESTION 16arrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 17.00 μmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. olo sample A B C D symbol 52 25 15 8 212 82 91 39 radionuclide Mn O Pb Y half-life 6.0 days 122. seconds 11. hours 59.0 days initial radioactivity (choose one) (choose one) (choose one) (choose one) > time for amount of radionuclide to decrease to 1/32 of initial amount X days seconds hours days Ararrow_forward
- = 1 = 2 = 3 = 4 = 5 = 6 = 7 = 8 = 9 -10 -11 Rewrite the following radioactive decay series. α བང 231 β 4. Pa 227 He 91 2 89 Ac α →> 18 Ararrow_forwardSome workers responding to the 1986 explosion at the Chernobyl nuclear plant absorbed 403 J of radiation primarily from γ rays. What is the effective dose (in Sv) absorbed by a 65.0 kg worker?arrow_forwardSearch for Life Transfer Task 1 - Poisoned Shopworkers Back in 1920, one of the most sought-after jobs on the East Coast was a dial painter at a watch factory. These workers were all female, and they absolutely loved their jobs. These girls were instructed to paint the number on a watch with paint laced with an element called radium. Years later, many of these workers developed many medical problems with their bones. Some girls lost their jaw bones, and others’ bones broke when they walked across a room. Several doctors thought that phosphorus poisoned the girls’ bones. Their companies insisted that radium was not damaging their bones in order to avoid a lawsuit. Answer the questions below to answer the big questions: Was it the radium or the phosphorus that poisoned the girls’ bones? Were the factories responsible for the girls’ sicknesses?arrow_forward
- Which of the following units is most commonly associated with the measurement of tissue damage caused by radiation? Gray Curie Bequerel Sievert Joulearrow_forward13. What is the missing particle? 2Am + He → 2¿n + B. 2Bk 243 95 A. 2Bk 244 97 245 97 C. 2Bk 246 97 D. 257BKarrow_forwardPart B Balance the decay equations Determine the particle that balances the equation.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY