
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
show-all-working-explaining-detailly-each-step
Answer should be typewritten using a computer keyboard.
![33.
Identify the conjugate acids in the following
acids-base equilibrium
[Fe(H2O)6]"(aq) + H2O(I) S
[Fe(H2O)s]?"(aq) + H3O°(aq)
H,O*(aq)
[Fe(H2O)}*(aq)
H2O(I)
2
3
ABCD](https://content.bartleby.com/qna-images/question/5a6d9c67-6f13-49d2-ac4d-2d996f90a88b/ba206263-8bfe-4348-b066-6175d481b251/mwhdwip_thumbnail.jpeg)
Transcribed Image Text:33.
Identify the conjugate acids in the following
acids-base equilibrium
[Fe(H2O)6]"(aq) + H2O(I) S
[Fe(H2O)s]?"(aq) + H3O°(aq)
H,O*(aq)
[Fe(H2O)}*(aq)
H2O(I)
2
3
ABCD
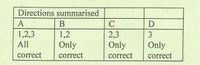
Transcribed Image Text:Directions summarised
A
B
1,2,3
1,2
2,3
Only
All
Only
Only
correct
correct
correct
correct
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- We want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forwardΣ * 00 T The aromatic compound C6H-NH, is best-referred to as: O a. Phenol O b. Aniline O c. Toluidine Od. Toluene e. Nitrobernzene Time left 1:28:48 Quiz navigation DELL Esc F1 F2 F3 F4 F5 F6. F7 F8 F10 i V 3. 4. 5. 7. qel Lock C. Altarrow_forwardpls solve this ques within 10-15 minutes I'll give you multiple upvotes.arrow_forward
- We want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forwardA small intermittent fraction is collected and discarded. This is a good practice because intermittent fractions contain unknown substances intermittent fractions contain substances synthesized during distillation intermittent fractions are composed of both unseparated components of the mixture intermittent fractions are toxic intermittent fractions decompose quickly over timearrow_forwardWe want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forward
- Pls help ASAP. Pls show all work and calculations.arrow_forward7arrow_forwardI need help with calculations i need help by step by step show the work please Below is a simple set of weights obtained while immersing potato slices in various sugar solutions over 30 minutes. a. For each solution, calculate the rate of weight change over this 30-minute period. . Once you calculate these rates - . Write your answer in standard notation and use 3 digits past the decimal point - e.g. 0.005, 0.030. (Note that the last zero in an answer is considered to be a digit). 0.0 M sucrose = ____________________ g/min 0.4 M sucrose = _____________________ g/min 0.6 M sucrose = _____________________ g/min 0.8 M sucrose = _____________________ g/min 1.0 M sucrose = _____________________ g/minarrow_forward
- We want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forwardQlaccd sign in - C = ctrl McGraw-Hill Ec X A ALEKS-Shusha X https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym 0 tab shift ↑ O CHEMICAL REACTIONS Calculating molarity using solute moles X C A chemist prepares a solution of silver(II) oxide (AgO) by measuring out 0.0121 μmol of silver(II) oxide into a 500. mL volumetric flask an filling the flask to the mark with water. mol caps lock AVILION Calculate the concentration in mol/L of the chemist's silver(II) oxide solution. Be sure your answer has the correct number of significant digits. L Explanation esc ALEKS K →1 fn Type here to search ? A Z X Check f2 2 W P Pavilion x360 hp erywhere and every way stream ORO S x10 # X 3 X alt * E 14 $ 4 с S ㅇ 발 % 5 V T G 6 mu 17 4+ & Y 7 H ALEKS hp 18 8 X © 2022 McGraw Hill LLC. All Rights Reserved. a 19 DII NM McGraw-Hill Ex A ALEKS-Shush x + 95T1680XL... K ❖ ho A") DDI f12 6:50 PM 7/22/2022 Terms of Use | Privacy Center Acces 87°F insert alt + [ 2/5 ?…arrow_forward11 IMPORTANT! Please adhere to the following conditions while completing this question: 1. Use only HIGH SCHOOL knowledge to complete the question. No University level workarounds. This is so that I can understand the response. 2. Respond using TYPED WORDS, please. I know this will be harder but you are allowed to use only tools and etc... 3. Keep your response organized. This is also important as your work will likely end up in my notes.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY