
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
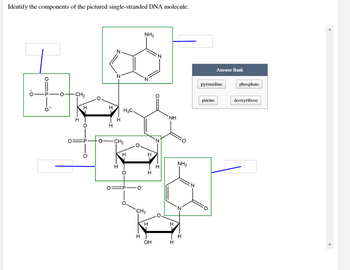
Transcribed Image Text:Identify the components of the pictured single-stranded DNA molecule.
DIO
-0-
-CH₂
H
H
H
N
H
H₂C
-CH₂
H
O
NH₂
CH₂
H
H
OH
N
H
NH
H
NH₂
'N'
I
Answer Bank
pyrimidine
purine
phosphate
deoxyribose
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If A pairs with T and G pairs with C solve this in a DNA the amount of T is 20% what is the amount of A ,G , C ?arrow_forwardGive the correct numbering of the rings. How is this structure different from sucrose? CICII. II 11 11 НО НО НО НО 11 H Н r r О 11 си и Is the polymer below alpha or beta anomer? Number the carbons 11 OH 11 11 01. 11 CHOH О СН OLL H H И О С-Н ОН OH CH₂OH 11 (FE сист What type of reaction is occurring? Oxidation/ reduction? What is the reagent used? 11 — If CHOH 11 сит 11 OH OH CH OH What reagent is used to form the product? HO Н II II ГНЕ HO-H CH OH U НО H H-- -H -OH H-OH CH OH HO-H H OH CH₂OHarrow_forwardEnzymatic DNA sequencing uses to cause termination of DNA polymerase. A. rNTP B. dideoxy NTP C. A and C D. deoxy NTParrow_forward
- mplified with different primer sets. QUESTION 7 Which of the following is true regarding wobble pairing? Wobble is possible due to the flexibility in the DNA helical structure Wobble is caused by chemically induced mutations Base analogs are incorporated due to wobble pairing The wild-type sequence of a gene may be altered during DNA synthesis due to wobble mis-pairing There is no basis or support for wobble pairing having a role in causing spontaneous mutations ick Save and Submit to save and submit. Click Save All Answers to save all answers.arrow_forward17 Provide the abbreviation of the following nucleotide. ofotored H OH OH d A G C T U MP DP TP deoxyribose NH NH₂arrow_forwardConsider the following DNA fragment. Identify the 5' and 3' ends, and label each of the nitrogenous basesarrow_forward
- On your Scantron, best possible answer for each question and fill in the corresponding bubble on your Scantron There are 50 total questions, each worth 3 points. 1. Which structure represents a nucleotide? A) CH,O- -O. 0- OH ОН B) NH2 CH, H H OH OH C) HO-CH, OH H H H. OH H H D) NH, 0. CH, OH OH 0-P=0 2. Which vitamin is fat soluble?arrow_forwardDuring replication, which strand is replicated in small fragments? A) right strand B) lagging strand C) left strand D) leading strand E) parent strandarrow_forward18. Based on the following nucleotide structure, answer the following questions: NH₂ R -OCH₂ a. OH OH Is the base present in this nucleotide a purine or a pyrimidine? b. What is the abbreviation for the base present in this nucleotide? (A, T, C, G, U) c. Would the sugar in this nucleotide be found in molecules of DNA or RNA or both? d. Would the base in this nucleotide be found in molecules of DNA, RNA or both? a baco:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY