
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Don't used hand raiting
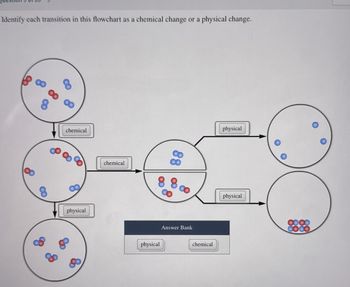
Transcribed Image Text:Identify each transition in this flowchart as a chemical change or a physical change.
8
88
8
chemical
8
8
physical
chemical
88.
Answer Bank
8
physical
physical
chemical
physical
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 17. Under certain circumstances, carbon dioxide, CO2(g)CO2(g), can be made to react with hydrogen gas, H2(g)H2(g), to produce methane, CH4(g)CH4(g), and water vapor, H2O(g)H2O(g): CO2(g)+4H2(g)→CH4(g)+2H2O(g) A. How many moles of methane are produced when 85.1 molesmoles of carbon dioxide gas react with excess hydrogen gas? Express your answer with the appropriate units. B. How many moles of hydrogen gas would be needed to react with excess carbon dioxide to produce 76.6 molesmoles of water vapor? Express your answer with the appropriate units.arrow_forward35R) AH for the reaction IF5 (g) → IF3 (g) + F2 (g) is kJ, give the data below. IF (g) + F2 (g) → IF3 (g) AH = -390 kJ IF (g) + 2F2 (g) → IF5 (g) AH =-745 kJ A) +355 B) -1135 C) +1135 D) +35 E) -35. vanAarrow_forwardU.S. MINT COIN Measured MASS m (grams) SIG. FIGS. Dime 2.268 Penny 2.500 Quarter 5.670 COIN Uncertainty in mass δm (grams) (taken as EXACT) SIG. FIGS. Dime 0.001 Penny 0.001 Quarter 0.001 ----------------------------- COIN Relative Uncertainty in mass δm/m (%) SIG. FIGS. Dime ? Penny ? Quarter ?arrow_forward
- For the reaction SbCl 5 (g) = SbCl 3 (g) Cl 2 (g); Delta G^ (SbCl 5 )=-334.34 k /mol; Delta G^ f(SbCl 3 )=-301.25 kJ/mol; Delta * H ^ 0 (SbC * l_{5}) = - 394.34kl / m * o * l; Delta H^ f (SbCl 3 )=-313.80 kJ/mol Calculate the value of the equilibrium constant (K_{p}) at 800 K and 1 atm pressure . 1.91 * 10 ^ 2 1.71 * 10 ^ 2 1.31 * 10 ^ 3 1.11 * 10 ^ 3 None of the abovearrow_forwardThe thermagram in Figure below shows the change in mass of a sample of Copper sulfate pentahydrate, CuSO++5H2O (249.5 g/mole) as a function of temperature. The original sample weighing 25.0 mg was heated from room temperature to 1000°C at a rate of 5º C per minute. The following changes in mass and corresponding temperature ranges were observed : Loss of 1.80 mg from 100 – 250° C. Loss of 7.212 mg from 350 – 550° C., Loss of 8.015 mg from 600 – 800° C. Determine the identities of the volatilization products and the solid residue at each step of the thermal decomposition. Given that: H=1 g/mol , S =32 g/mol , 0=16 g/mol. 20.00 - 15.00 10.00 500- 0.00 400 s00 600 700 Temperature C) 100 200 300 00 s00 1000 Thermogram for CUSO4.5H20arrow_forwardI don't reallt understand. I know I'm supposed to switch the sign but I don't see that reaction on the table. Keep in mind sig figs too. TYSM :)arrow_forward
- I am not sure how to answer this. Can you help? Thanks!arrow_forwardplease use the photo attached for the informationarrow_forwardCould someone Please Help Me with this! No Plagirism Please! 3 · Provide at least 1 example of a reversible process (either physical or chemical). What has to be done to the process to make it reverse? Part 2—Copper (II) Chloride System - Cu(H2O)62+(aq) + 4Cl–(aq) + heat ⇆ CuCl42–(aq) + 6H2O(l) Blue Green Test Tube Procedure (Impacts) Predictions Observations (Result/Shift) 1 No tests (control) 2 Add 4M NaCl dropwise until change is observed *increases concentration of Cl─ in the system 3 Add 0.05M AgNO3 drop-wise until change is observed *decreases concentration of Cl─ in the system 4 Add H2O dropwise until change is observed *increases concentration of H2O in the system 5 Add acetone dropwise until change is observed *decreases concentration of H2O in the system 6 Place in hot water bath for 1-2 minutes *adds heat to the reaction system…arrow_forward
- Convert 17.3 JJ to Calories 132 kJkJ to Calories 56.2 CalCal to joules.arrow_forwardChoose all of the processes from below which describe changes which are independent of the path by which the change occurs. 1. the elevation increase experienced by a traveller travelling from Grand Isle, LA to Denver, Colorado2. the kinetic energy aquired by a bullet as it reaches a specific speed3. the heat generated by a car engine as it burns a gallon of gasoline4. the heat evolved when a cube of sugar is oxidized to CO2(g) and H2O(g)5. the work generated by a homogeneous gaseous chemical reaction carried out inside of a bomb calorimeter6. the odometer mileage increase observed by a traveller travelling from Louisiana to Canada7. the latitude increase experienced by a traveller travelling from Baton Rouge, LA to Anchorage, Alaskaarrow_forwardA student dissolves 13.7 g of sodium chloride (NaCl) in 300. g of water in a well-insulated open cup. She then observes the temperature of the water fall from 21.0 °C to 20.4 °C over the course of 6.9 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NaCl(s) → Na' (aq) + Cl (aq) olo You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 1 significant digit. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. O exothermic Is this reaction exothermic, endothermic, or neither? O endothermic O neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. I kJ kJ Calculate the reaction…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER