
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
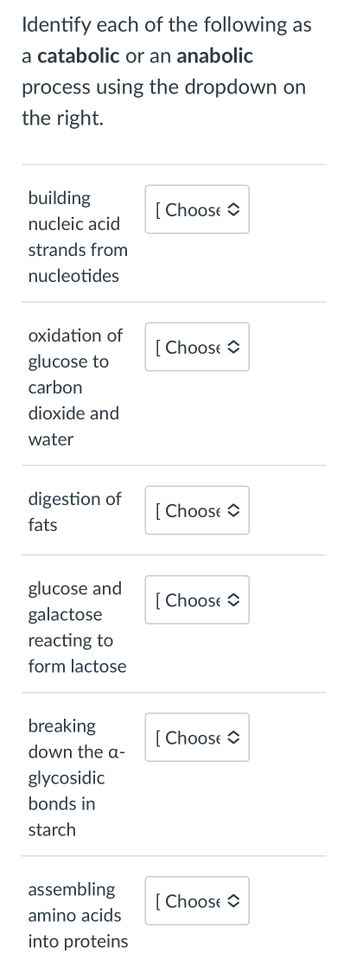
Transcribed Image Text:Identify each of the following as
a catabolic or an anabolic
process using the dropdown on
the right.
building
nucleic acid
strands from
nucleotides
oxidation of
glucose to
carbon
dioxide and
water
digestion of
fats
glucose and
galactose
reacting to
form lactose
breaking
down the a-
glycosidic
bonds in
starch
assembling
amino acids
into proteins
[Choose
[Choose
[Choose
[Choose
[Choose
[ Choose
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- We are consuming a lot of polymers in our foods and only some monomers. The polymers we ingest must be converted into monomers before being absorbed, with a few exceptions that we won't discuss. Amylase fatty acid nucleotide amino acid ribonuclease peptide polysaccharide triglyceride trypsin monosaccharide RNA lipase Fill the table. Ise the word bank above to fill-in the columns: macronutrient polymer Enzyme that hydrolyzes polymer into monomer monomer Proteins amino acid Carbohydrates monosaccharide Lipids fatty acid Nucleic acidsarrow_forwardThe breakdown of sugar involves what type of reaction?arrow_forward1) Describe the change of Cis-aconitate to Isocitrate. 2) Are enzymes changed during a reaction they catalyze? 3) What does "induced fit" mean when talking about enzymesarrow_forward
- What process is taking place in the illustration? Supply the appropriate labels for all numbered parts of the illustration.arrow_forwardUse the diagram to answer the following questions. What is the name of the process that occurs at X? What is the name of the process that occurs at Y? What is the molecule labeled D? What is the part of the molecule labeled F?arrow_forwardThe production of Ribose 5-phosphate in the Pentose Phosphate Pathway is needed in the synthesis of which biomolecule? Group of answer choices Carbohydrate Synthesis Fatty Acid Synthesis Nucleic Acid Synthesis Protein synthesisarrow_forward
- The most important high-energy bond in a molecule of ATP is between: O A) the nitrogenous base and the first phosphate group O B) the ribose sugar and the nitrogenous base O C) the second and third phosphate groups O D) the ribose sugar and the first phosphate grouparrow_forwardMatch the antibiotic to its target below tetracycline, macrolides, aminoglycosides [Choose ] [Choose ] ciprofloxacin, a fluoroquinolone azoles bacterial enzymes involved in folic acid synthesis rifampin, a rifamycin Bacterial RNA Polymerase Bacterial Gyrase trimethoprim, sulfamethoxazole methicillin penicillin 70S ribosomes Question 52arrow_forwardAlthough the first two carbons of fructose and glucose are identical in structure to DHAP and GADP (from glycolysis), DHAP and GADP equilibriate on their in solution to favor the ketone over the aldehyde, while fructose and glucose do not. Why? a)The larger size of the molecule sterically hinders the isomerization b)The larger sugars have more OH groups which hydrogen bond and disrupt isomerization c)The larger sugars cyclize, and there is no carbonyl to isomerize in the cyclic form d)The larger sugars cyclize, and in the cyclic form the hydrogen bonding is very strong e)The larger sugars are less soluble in water than the smaller sugarsarrow_forward
- The lipid shown here is produced by certain microorganisms that live in extremely warm environments, such as hot springs. Assuming that this lipid makes up a portion of the organism’s membrane lipids, would the organisms produce more of this lipid or less of this lipid as the temperature increased from 45°C to 65°C?arrow_forward18arrow_forwardClassify the monosaccharides. H H-C- -OH H-C- -OH H- CH₂OH D-erythrose H- CH₂OH FO -OH H. CH₂OH D-erythrulose H- -OH -OH H-C OH CH₂OH H- D-ribose CH₂OH OH H-C OH CH₂OH D-ribulose H H-C- CH₂OH D-glyceraldehyde -OH HO- -C- H- CH₂OH FO -H -C- -OH H-C OH CH₂OH D-fructose CH₂OH C=O CH₂OH Dihydroxyacetone triose Answer Bank ketose hexose aldose tetrose pentosearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON