
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please show your work (THIS IS NOT A GRADED ASSIGNMENT)
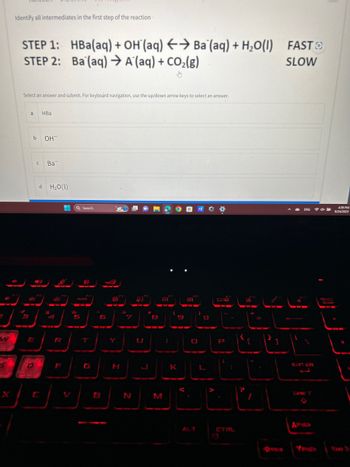
Transcribed Image Text:Identify all intermediates in the first step of the reaction
5
STEP 1: HBa(aq) + OH(aq) → Ba (aq) + H₂O(1) FAST
STEP 2: Ba (aq) → A (aq) + CO₂(g)
SLOW
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
a HBa
b
J
E
D
OH
с Ba
d H₂O(1)
+ Q Search
P
AURA
5
A
6
Je
Y
H
N
8
I
M
90
K
503
IA
ALT
0/5
{
[
Baget
L
P
CTRL
PA
P
ENG
ENTER
APOL
dx la
4:39 PM
9/24/2023
b
Expert Solution
arrow_forward
Step 1: Formula
To get the overall reaction, add all the steps of the reaction mechanism.
In the reaction mechanism only reactants and products are present and intermediates cancel out.
In this way we can determine the intermediate species, species which are not present in the overall reaction are the intermediate species.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Need help on the following please.arrow_forwardOne of the first drugs to be approved for use in treatment of acquired immune deficiency syndrome (AIDS) was azidothymidine (AZT). CH3 C6 || Cs- H. N5 C8- H H. H-O- H C4 H C2-C3 H N1 N3arrow_forwardtoSave Paste Home Insert Draw Design Layout References Mailings Review View Help X US S Clipboard F 1 ge 2 of 4 1 4. Font Problem Set 5 - Chapter 5 Paragraph 2 A Styles 24 of 491 words Styles F Editing 3 Dictate Text Predictions: On Voice N: (g) + O2(g) → 2 NO (R) AH--180.5 kJ N2(g) + 3 H2(g) → 2 NHI(g) AH = -91.8 kJ 2 H2(g) + O2(g) → 2 H₂O (g) AH = -483.6 kJ Aidan Tomlinson AT 4 3. Calculate AH for the reaction 4 NO (g) + 6 H2O (g) →4 NH3(g) + 5 O: (g), from the following data. Editor Calculate AH for the reaction 2 Al (s) + 3 Ch (g) 2 AICI (s). given: 2 Al (s) + 6 HCl(aq) → 2 AICh (aq) + 3 H2(g) AH-1049. kJ HCI (g) → HCl (as) H: (g) + Ch (g) → 2 HCl (g) AICE (s) → AICia (94) AH--74.8 kJ AH-1845. kJ AH = -323. kl Focus F12 Reuse Files Editor Reuse Files FA 5 Editin 10:35 10/29/2 DELEarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY