
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
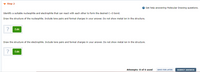
Transcribed Image Text:v Step 2
O Get help answering Molecular Drawing questions.
Identify a suitable nucleophile and electrophile that can react with each other to form the desired C-0 bond.
Draw the structure of the nucleophile. Include lone pairs and formal charges in your answer. Do not show metal ion in the structure.
Edit
Draw the structure of the electrophile. Include lone pairs and formal charges in your answer. Do not show metal ion in the structure.
?
Edit
Attempts: 0 of 6 used
SAVE FOR LATER
SUBMIT ANSWER

Transcribed Image Text:Review of Skills - Skill Builder 07.08
Provide a synthesis for the target molecule shown below, starting with compounds that contain no more than two carbon atoms. Show your retrosynthetic analysis, and then provide
a complete synthesis, showing all necessary reagents.
v Step 1
V Your answer is correct.
Identify a bond that can be readily made via a substitution reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Synthesise the target molecules drawn below using precursor chemicals within the following limitations: 1) ALL intermediates and reagents must be shown.2) Each molecule requires multiple step synthesis.3) You must also synthesize any organometallic reagents or ligands that you wish to use. b) Use any precursor molecules with 7 carbon atoms or less.arrow_forwardplease answer in text form and in proper format answer with must explanation , calculation for each part and steps clearlyarrow_forwardFraw the lower and higher molecular weightarrow_forward
- Co X Smartwork5 https://digital.wwnorton.com/46255 Srd attempt P X H- Organic Chemistry, X Draw the organic product of the reaction. 15 OF 18 QUESTIONS COMPLETED D21 Chapter <15arrow_forwardOrganic Chemistry Peter Vollhardt and Neil Schore Rank the compounds in order of decreasing reactivity towards electrophilic aromatic substitution. H3C- 0 COOH H3C- 0 Most reactive Least reactive Answer Bank -CH3 W.H. Freeman and Company presented by Macmillan Learning HOOC COOHarrow_forwardPropose a mechanism, draw and show all arrows correctly.arrow_forward
- Synthesise the target molecules drawn below using precursor chemicals within the following limitations: 1) ALL intermediates and reagents must be shown.2) Each molecule requires multiple step synthesis.3) You must also synthesize any organometallic reagents or ligands that you wish to use. a) Aspirin is a common pain killer that was developed by Bayer.arrow_forwardShow using resonance structures why the following structure adds meta and not ortho/para. NH3 Propose a mechanism for the following reaction. Remember, the aromatic ring will attack any positive charge- even one formed by water leaving! But rearrange first! OH H3CO. H3CO H2SO4arrow_forwardDo not give handwriting solution.arrow_forward
- Propose reagents and conditions for the following multistep syntheses. You may use any reagents necessary. Be sure to draw all isolable intermediates. Br. H.arrow_forwardSeveral reagents and several organic structures are shown below. Construct a multistep synthetic route for the synthesis of acetylene from ethane by dragging the appropriate pieces into the bins. Note that each bin will hold only one item, and not every given reagent or structure will be used. (Stoichiometry is omitted.)arrow_forwardFor the reaction sequence below, provide the correct reagents, intermediate or final product. Br You? Na enant.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning