
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
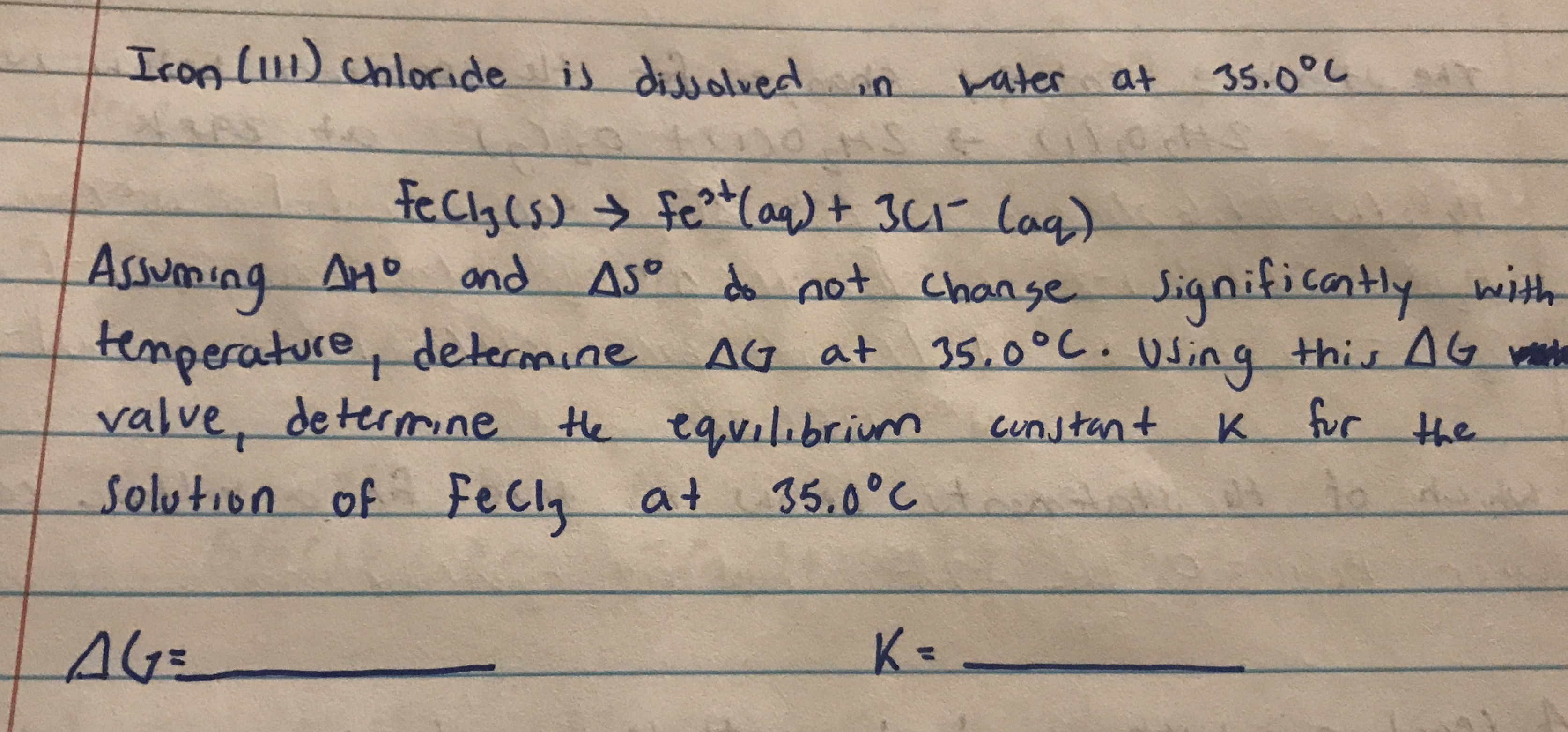
Transcribed Image Text:IconL11) chloride is
dissolvedin
rater at
35.0°し
fecyls) fe(aq)+ 3C1 lag)
Assuming Ano and
temperature, determine AG at
valve, determne
Solution of FeCl, at
AMD
AS° d not Change
Significantly with
35.0°C. q this AG
Using
the equilibrium
cunstant
K fur the
35.0°C
K=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Similar questions
- I need help with part c. I included the rest of the problem incase additianal info is needed. Consider the solubility of calcium oxalate (oxalate = C2O42-) in water: CaC2O4(s) ⟺ Ca2+(aq) + C2O42-(aq) a) Write the equilibrium expression for the reaction as written. b) Calculate the [Ca2+] from the dissolution of calcium oxalate in pure water. pKsp = 7.90 c) Calculate the [Ca2+] from the dissolution of calcium oxalate in a solution that contains 0.250 M ammonium oxalate.arrow_forwardPlease help mearrow_forwardSubmit Part B Consider the reaction, which takes place at a certain elevated temperature CO(g) + NH3(g)= HCONH2 (g), K. = 0.880 If a reaction vessel initially contains only C0 and NH3 at concentrations of 1.00 M and 2.00 M, respectively, what will the concentration of HCONH2 be at equilibrium? Express your answer with the appropriate units. • View Available Hint(s) HÀ [HCONH2] = Value Units Submit Next> Provide Feedback MacBook Airarrow_forward
- FOP Practice 15.7 - Enhanced with Feedback Review | Constants Part A Consider the reaction and its equilibrium constant: N2O4(g) = 2NO2(g) K. = 5.85 x 10 (at some temperature) Calculate Qc at the given concentrations. -3 Express the reaction quotient to three significant figures. A reaction mixture contains [NO2] = 0.0242 M and [N2O4] 0.0350 M. ΑΣφ You may want to reference (Pages 653 - 656) Section 15.7 while completing this problem. Qc =arrow_forward21. When the system is not at equilibrium, the reaction quotient Q for any reaction ... (A) will indicate if more reactants or products will be made. (B) will go up or down as determined by the equilibrium constant (C) will change over time until it is equal to the equilibrium constant. (D) will depend on the reaction stoichiometry. (E) All of the above.arrow_forwardHe etto Consider the Following reactions and their assoclated equilibrium constants': AtaBS c c sotE tor the eaction At 2B S D+E, haviny equilibriam constants kun CA) Kc=Kitkz (B) kc=K,/Kz (C)ku=Ki-Kz DJ Kc=CKi) CKa) (E) Kc=Kz/K.arrow_forward
- Given these initial concentrations: [SCN] = 0.000644 M, [Fe°'] 2+. = 0.000505 M, and [Fe(SCN)“"] = 0.000 M, determine the value 2+. for Kif the [Fe(SCN)] at equilibrium is 0.0000856 M. Answer:arrow_forwardCO to Withdr... Forms | Office of t... Content $ 4 Submit Answer 000 DOD F4 R The equilibrium constant, Kc, for the following reaction is 5.10 x 10-6 at 548 K. NH4Cl(s) NH3(g) + HCl(g) F X % Show Hint 5 If an equilibrium mixture of the three compounds in a 4.30 L container at 548 K contains 1.72 mol of NH4Cl(s) and 0.323 mol of NH3, the number of moles of HCl present is mol. 2 Bh Salinger Pages T KB Viewing Your Aca... Have F5 Retry Entire Group 9 more group attempts remaining [Review Topics] [References] Use the References to access important values if needed for this question. Cengage Learning Cengage Technical Support A X OWLv2 | Online X t 6 - in... Have Changes in... Scholarship Ameri... Changes MacBook Air F6 Y G H & 7 F7 U * 8 ► 11 F8 Inbox (470) - van X ( 9 K ►► F9 bio 1108 chat O F10 P Account Summar 4) Tp SAVAGE X FENTY... Previous F11 11 + = { ļ Next> Mon Oct 24 11 Save and Exit F12 11 } + Upda del-arrow_forwardCopper(I) ions in aqueous solution react with NH, (aq) according to Cu*(aq) + 2 NH,(aq) Cu(NH,)* (aq) Kf = 6.3 x 1010 %3D Calculate the solubility (in g-L-l) of CuBr(s) (Kp = 6.3 × 10-9) in 0.73 M NH, (aq). 14.54 solubility of CuBr(s): g/L Incorrectarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY