
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
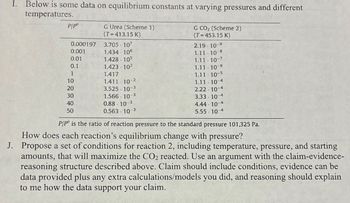
Transcribed Image Text:I. Below is some data on equilibrium constants at varying pressures and different
temperatures.
P/P
0.000197
0.001
0.01
0.1
1
10
20
30
40
50
G Urea (Scheme 1)
(T=413.15 K)
3.705-107
1.434 106
1.428.105
1.423 10²
1.417
1.411-10-²
3.525-10-3
1.566-10-3
0.88-10-3
0.563 10-3
G CO₂ (Scheme 2)
(T=453.15 K)
2.19 10-9
1.11.10-8
1.11.10-7
1.11.10-6
1.11.10-5
1.11.10 4
2.22-10-4
3.33 10 4
4.44-10-4
5.55-10-4
P/P is the ratio of reaction pressure to the standard pressure 101,325 Pa.
How does each reaction's equilibrium change with pressure?
J. Propose a set of conditions for reaction 2, including temperature, pressure, and starting
amounts, that will maximize the CO₂ reacted. Use an argument with the claim-evidence-
reasoning structure described above. Claim should include conditions, evidence can be
data provided plus any extra calculations/models you did, and reasoning should explain
to me how the data support your claim.
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Please provide detailed solution...arrow_forwardHow could I find this using the given information ?arrow_forwardFor the reaction system Fe(s) + H2 O(g)= FeO(s) + H2 (9) which has already reached a state of equilibrium, predict the effect that each of the following changes will have on the position of the equilibrium. Tell whether the equilibrium will shift to the right, will shift to the left, or will not be affected. a The pressure of hydrogen is increased by injecting an additional mole of hydrogen gas into the reaction vessel. shifts left shifts right no effect b Hydrogen gas is removed as it forms by use of a chemical absorbent, or "scrubber." shifts left shifts right no effect C An additional amount of solid iron is added to the reaction vessel. shifts left shifts right no effectarrow_forward
- Consider the reaction: P(s) + 3/2Cl₂ (9) ⇒ PCls (g) Write the equilibrium constant for this reaction in terms of the equilibrium constants, K₁ and K₂, for the reactions below: $ 4 P(s) +5/2C1₂ (9) PC15 (9) K1 PC13 (9) + Cl₂ (g) =PC15 (9) K₂ Submit Answer For answers with both a subscript and a superscript, enter the subscript first. For example, enter K? If the first equilibrium constant should be squared. K = 000 000 F4 R F Show Hint % 5 Retry Entire Group 9 more group attempts remaining [Review Topics] [References] Use the References to access important values if needed for this question. F5 T Cengage Learning Cengage Technical Support G A 6 MacBook Air F6 Y H & ✓ 7 ◄◄ F7 U * 8 J F8 ( 9 ►► K F9 O ) DIO 1108 chat 0 L F10 - P - ^ Previous F11 SAVAGE X FENTY... { + = Next> Save and Exit F12 11 Garrow_forwardConsider the reaction: 12(g) =21(g) The equilibrium constant, Kc, for this reaction is 3.76 x 10-3 at T = 1000 K. Initially, 1.36 moles of 12 is placed into a 5.00 L reaction flask at T = 1000 K. What is the equilibrium concentration of I? Input your answer in units of M (molarity) to 3 decimal places. Your Answer: 4arrow_forwardA mixture of ammonia, nitrogen, and hydrogen was allowed to come to equilibrium at 900K. 2NH3 ⇌ 2H2 + N2 Kc = 0.0076. An analysis of the mixture at equilibrium revealed 0.31 M of H2 and 1.8 M of NH3. Determine the equilibrium concentration of N2 (in M) in this mixture. (use 2 significant figures)arrow_forward
- Calculate the value of Kc.arrow_forwardFor the reaction 12(g) +Br₂(g) →21Br(g) Ke=280 at 150 °C. Suppose that 0.520 mol IBr in a 2.00-L flask is allowed to reach equilibrium at 150 °C. Esc Type here to search WE in R ▼ What is the equilibrium concentration of I₂? Express your answer in moles per liter to three signifi IVE ΑΣΦ [12] = 0.012 Submit Previous Answers Request Answer * Incorrect; Try Again; 7 attempts remaining Part C What is the equilibrium concentration of Br₂? Express your answer in moles per liter to three significant figu ΕΧΕΙ ΑΣΦ Br₂ = 0.0120 Submit Previous Answers Request Answer ? 8 Marrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY