
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Use the second picture to answer the qu

Transcribed Image Text:8.
Question:
Chapter: 1 Lesson: 7
I. Based on Figure F01-7-6, what percentage of the incoming solar energy is reflected out of the atmosphere without being
absorbed?
II. What happens to the remainder of the incoming solar radiation? What percentage of the total solar radiation is absorbed by
the earth?
Hint:
What do the yellow arrows in the figure depict? What do the orange arrows depict?
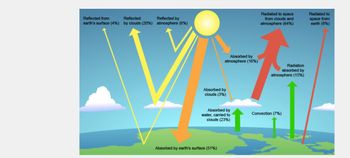
Transcribed Image Text:Reflected from
Reflected
Reflected by
earth's surface (4%) by clouds (20%) atmosphere (6%)
V
Absorbed by
atmosphere (16%)
Absorbed by
clouds (3%)
Absorbed by
water, carried to
clouds (23%)
Absorbed by earth's surface (51%)
Radiated to space
from clouds and
atmosphere (64%)
Radiation
absorbed by
atmosphere (15%)
Convection (7%)
Radiated to
space from
earth (6%)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which statistical distribution is commonly used to model continuous data that follow a normal distribution? a) Poisson distribution b) Exponential distribution c) Normal distribution d) Binomial distributionarrow_forwardplease solve question 44 thannnkssarrow_forwardTransitions states occur at a saddle point. True or False. Why?arrow_forward
- Determine the lowest value od n for which m1 can (theoretically) have a value of +4.arrow_forwardCould you explain how you got R for (c)?arrow_forwardNow draw two waves on your own sheet of paper. Predict what would happen if they arrive at the same spot at the same time (also known as "in-phase"). What would be the effect? Enter your answer here Save Answerarrow_forward
- why Step 3 and 4 are both for coefficient for ψ3 ?????arrow_forward*What type of system is best modeled by deterministic equations?* a) Chaotic systems b) Systems with noise c) Predictable systems d) Random systemsarrow_forwardWhat is the total spin of copper atom in its standard electron configuration? (Chemistry)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY