
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Please help
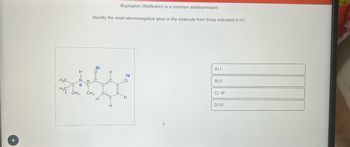
Transcribed Image Text:+
I-Z=
H3C.
нас онз
Bupropion (Wellbutrin) is a common antidepressant.
Identify the most electronegative atom in the molecule from those indicated (I-IV).
=o=0
CH3
IV
H
A) I
B) II
C) III
D) IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Materials Needed solid I2 solid CUSO4-5H20 food dye solid (NH4)2SO4 heavy metals waste container halogenated waste container non-halogenated waste container semi-micro test tubes and rack regular test tubes and rack squash pipettes acetone cyclohexane propan-2-ol Method Part A: Solubility of ionic and molecular solids 1. Place a small amount (about the size of 1 grain of rice, see picture) of copper sulfate into each of three DRY semi-micro test tubes. Add 20 drops of water to the first test tube and gently flick the test tube with your finger to ensure mixing. 2. Repeat step 1 using acetone in place of water as the solvent in the second test tube. 3. Repeat step 2 replacing acetone with cyclohexane in the third test tube. Hold the test tubes against a white background to compare the solubility of copper sulfate in the three solvents and record your results. Discard these mixtures into the heavy metals waste container in the fume cupboard. Once these test tubes have been emptied you…arrow_forwardHello! Could you please answer this with one long dimentional analysis like I am supposed to do it? Thank you!arrow_forward16. A physician orders that a child should be given potassium chloride per kilogram (mEq/kg). The child weighs 675 oz. 1.6 milliequivalents NDC 00000-0000-00 POTASSIUM CHLORIDE For Injection Concentrate, USP 40 mEq (2 mEq/mL)) 20 mL Single Dose Vial Rx only DCL DELMAR Cengage Learning For Educational Purposes Only a. What dosage in mEq should the child receive? (Round off to near- est whole number.) b. If the medication is diluted correctly, how many mL should be injected? 00-0000-00000 Compiled loom Temperat S225C 77 USP Cengage Learning 2013 Exp. Date Lot.arrow_forward
- how do you this? how to enter it in the calculator?arrow_forwardWhat volume, in ml, of 0.5000M HCN can be made from 16.28ml of 14.37M HCN? A Moving to another question will save this response. chm1045l quiz6 2.pdf UI Online - Doc_....pdf AcroRdrDC_21 MAR 10 3 átv W MacBook Airarrow_forwardPlease check my answerarrow_forward
- Matti is given two bottles of copper (II) sulfate solutions in her senior high chemistry lab. She is told that one bottle contains a saturated solution and the other one contains an unsaturated solution. The solutions are the exact same color and have no particles in the bottles. Describe what Matti can do to distinguish between the two solutions. A- 米 U X2 x? #3 X Next page age You are logged in as Kaylee Krys (Log out) VLL Chemistry CP 2021-2022 (Fickett). Get the mobile app MacBook Air F8 O00 F4 F5 F6 F2 F3 & * @ # $ 7 9 2 3 4 Q W E R T Y А S D F G H M Y Barrow_forwardPlease helparrow_forwardAssay 5 KB) Page of 2 - ZOOM + 3. If a researcher starts with 3.00 mL of a 4.50 mg/mL protein solution and adds 10.0 mL of water, what is the amount (mg) of protein in the diluted solution? Next Previous awearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY