
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I would need help finding the average molarity
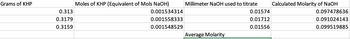
Transcribed Image Text:Grams of KHP
0.313
0.3179
0.3159
Moles of KHP (Equivalent of Mols NaOH)
0.001534314
0.001558333
0.001548529
Millimeter NaOH used to titrate
Average Molarity
0.01574
0.01712
0.01556
Calculated Molarity of NaOH
0.097478636
0.091024143
0.099519885
Expert Solution
arrow_forward
Step 1
The average of a set of numbers is simply the sum of the numbers divided by the total number of values in the set.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please Answer this question in the same format thats in the picture below on paper, answer correctly. Question: Calculate the number of moles in: 13.5 g magnesium nitrate [Mg(NO3)2]arrow_forwardIf I have 0.924 moles of a 0.376 M solution, what volume of that solution do I have in L?arrow_forwardStandardization of a sodium hydroxide solution against potassium hydrogen phthalate (KHP) yielded the accompanying results Mass of KHP.g 0.7987 0 8365 0.8104 0.8039 Volume of NaOH, mL 38.29 39.96 38.51 38.29 Calculate the average molarity of the basearrow_forward
- Write an equation that shows the mathematical relationship using M for molarity, L for volume in liters, and molesarrow_forwardIf 3.18 g BaCl2 is dissolved in enough solvent to make 500. mL of solution, what is the molarity of this solution?arrow_forwardAnswer this question in the same format thats in the picture below on paper. Answer correctly. Question: Calculate the number of moles in: 0.50 g sodium bromide (NaBr)arrow_forward
- If 0.0458 mol CaCl20.0458 mol CaCl2 is dissolved in water to make a 0.490 M0.490 M solution, what is the volume of the solution?arrow_forwardA 4.07 g sample of a laboratory solution contains 1.85 g of acid. What is the concentration of the solution as a mass percentage?arrow_forwardhow many milliliters of 0.659 m h3po4 will contain 0.12 mol h3po4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY