
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
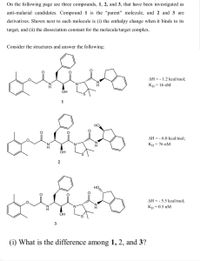
Transcribed Image Text:On the following page are three compounds, 1, 2, and 3, that have been investigated as
anti-malarial candidates. Compound 1 is the "parent" molecule, and 2 and 3 are
derivatives. Shown next to each molecule is (i) the enthalpy change when it binds to its
target, and (ii) the dissociation constant for the molecule/target complex.
Consider the structures and answer the following:
AH = - 1.2 kcal/mol;
Kp = 16 nM
OH
1
HO
AH = - 6.0 kcal/mol;
Kp = 76 nM
OH
AH = - 5.5 kcal/mol;
Kp = 0.5 nM
OH
3
(i) What is the difference among 1, 2, and 3?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How does the pKe of the conjugate acid of benzylamine (CgH5CH,NH2) compare to the pk,'s of the conjugate acids of cyclohexanamine (10.7) and aniline (4.6)? Explain your choice.arrow_forwardDetermining if a Base Is Strong Enough to Deprotonate an Acid Which of the following bases is strong enough to deprotonate N,Ndimethylacetamide [CH3CON(CH3)2, pKa = 30], so that equilibrium favors the products:(a)NaNH2; (b) NaOH?arrow_forwardExample: Sulfacetamide degradation presents a pH-rate profile U-shaped. The first order constant in the pH independent zone (5-11) is 9 x 10-6 s-1 at 120° C. The activation energy is 22.9 kcal mol -1 at pH 7.4. Calculate the retest period at 25° C. R= 8,314 JK-¹mol-1 1 cal = 4,1868 J H₂N ZIarrow_forward
- Please don't provide handwritten solutionarrow_forward) The potency of antagonists is frequently defined in terms of either their IC50, ortheir Ki. Explain what these two terms mean.arrow_forwardDepending on the size and complexity of the molecule, small chemical alterations can impart significant activity differences, especially if a certain part of the molecule is critical for binding. Considering this, select structure(s) below that are expected to have high cholinergic agonist activity based on what we learned about acetylcholine's SAR.arrow_forward
- Which of the following bases are strong enough to deprotonate C6H5OH (pKa = 10) so that equilibrium favors the products: (a) H2O; (b) NaOH; (c) NaNH2; (d) CH3NH2; (e) NaHCO3; (f) NaSH; (g) NaH?arrow_forwardWhich of the following bases are strong enough to deprotonate C6H5OH(pKa = 10) so that equilibrium favors the products:(a) H2O; (b) NaOH; (c) NaNH2; (d) CH3NH2; (e) NaHCO3; (f) NaSH; (g)NaH?arrow_forwardM8arrow_forward
- If the EC50 of an agonist is 3 x 10-7 mol/L and the presence of antagonist changes this EC50 to 3 x 10-6 mol/L where the Emax stays unaffected. Pertaining to the agonist - antagonist interaction, which of the following statement(s) do you think are correct? Group of answer choices A.competitive reversible; log (rA-1) = 0.95 B.competitive irreversible; log (rA-1) = 0.95 C.non-competitive irreversible; log (rA-1) = 0.95 D.competitive reversible; log (rA-1) = 10 E.competitive reversible; log (rA-1) = 9.5arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardConjugate base acid Iso-Butyronitrile pka_ → 1) Consider the reaction AH(+) + H₂O A:+H3O+. For the following named acids: 1) draw the structure of the acid, 2) give the approximate pKa of the conjugate acid (±1 pKa unit), 3) give the name of the conjugate base, and 4) draw the structure of the conjugate base. For the purposes of answering this question you may use pKa values evenly divisible by 5. N-methylimidazolium cation pka_ Triethyl phosphonoacetate pKa _ acid N-Ethylcarbamic pka_arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning