
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
I want to know the process of interpolation of H2' .
How can I do this with superheated steam table? can you show me the exact process?
I don't know how to interpolation it from the steam table ( I don't know what values to use)
Here's a steam table. Could you show me the process of selecting value to interpolating?
https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Flearncheme.com%2Fwp-content%2Fuploads%2F2022%2F11%2FSteam_Tables_1.21.xlsx&wdOrigin=BROWSELINK
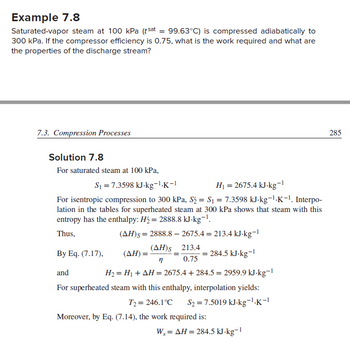
Transcribed Image Text:Example 7.8
Saturated-vapor steam at 100 kPa (tsat = 99.63°C) is compressed adiabatically to
300 kPa. If the compressor efficiency is 0.75, what is the work required and what are
the properties of the discharge stream?
7.3. Compression Processes
Solution 7.8
For saturated steam at 100 kPa,
S₁ =7.3598 kJ-kg-¹.K-1
For isentropic compression to 300 kPa, S₂ = S₁ = 7.3598 kJ-kg-¹.K-¹. Interpo-
lation in the tables for superheated steam at 300 kPa shows that steam with this
entropy has the enthalpy: H₂ = 2888.8 kJ-kg-¹.
Thus,
(AH)s=2888.8 - 2675.4 = 213.4 kJ-kg-1
H₁ = 2675.4 kJ-kg-¹
-1
(ΔΗ), 213.4
n
0.75
H₂ = H₁ + AH = 2675.4+284.5 = 2959.9 kJ-kg-1
(ΔΗ) = ·
By Eq. (7.17),
and
For superheated steam with this enthalpy, interpolation yields:
T2 = 246.1°C S₂ = 7.5019 kJ-kg-¹.K-1
= 284.5 kJ-kg-¹
Moreover, by Eq. (7.14), the work required is:
W, = AH = 284.5 kJ-kg-¹
285
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 1 images

Knowledge Booster
Similar questions
- Show me the balances for each subsequent system: overall, air conditioner, mixing point, and splitting point. Material balancesarrow_forwardANSWER QUESTION 5-19 – You need to try different orders of separation units. If you try only one order, then you need to justify why you didn’t try other orders of separation units. (I would highly recommend trying at least 3 combinations of order of separation units.)arrow_forwardD1.arrow_forward
- s] Type the answer (value only) in the boxes below for the following questions given the equation below: 2 C₂H6 (g) + 7 02 (g) 6 H₂0 (g) + 4 CO₂ (g) a) When [CO₂] is increasing at 0.32 mol/L.s, how fast is [C₂H6] decreasing? mol/L.s b) When [0₂] is decreasing at 0.28 mol/L.s, how fast is [H₂0] increasing? mol/L.s.arrow_forwardPlease I need solutions to this past question,I need it before 8:00pm WSTarrow_forwardA digital watch battery draws 0.20 milliamperes of current, which is provided by a mercury battery whose net reaction is: HgO(s) + Zn(s) → ZnO(s) + Hg(l) If a partially used battery contains 1.20 g of each of these four substances, for how many hours will the watch continue to run?arrow_forward
- Please refer to the photo attached below. It has the questions:)arrow_forwardThis problem is (6.21) from a book "Thermodynamics and Statistical Mechanics An Integrated Approach by M. Scott Shell"arrow_forwardThe fraction recrystallized-time data for the recrystallization at 350°C of a previously deformed aluminum are tabulated here. Assuming that the kinetics of this process obey the Avrami relationship, determine the fraction recrystallized after a total time of 116.8 min. Assume y1 = 0.38 and y, = 0.73. Fraction Recrystallized Time (min) 95.2 126.6 iarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The