
I need help with the steps of this practice problem. Can I get help to solve it ?
A piston cylinder device contains 0.8 kg of air initially at 100 kPa, 27 °C. The air is now compressed slowly in a polytropic process during which PV1.3=constant, until the volume reaches 1/2 its original volume. The system is then heated at constant pressure until the volume gets back to its original value. NOTE: THIS IS NOT A CYCLE.
Data: Rair = 0.287 kJ/kg K; Cv=0.731 kJ/kg K; Cp=1.018 kJ/kgK.
-
a) DeterminetheamountsANDdirectionsoftheWorkandHeat(each in kJ) for each of the two processes. SHOW ALL WORK used to get your values.
-
b) Sketch the two processes on a P-v diagram, clearly showing all three state points and the two processes with their directions.
Consider the diagram shown below for the given processes.
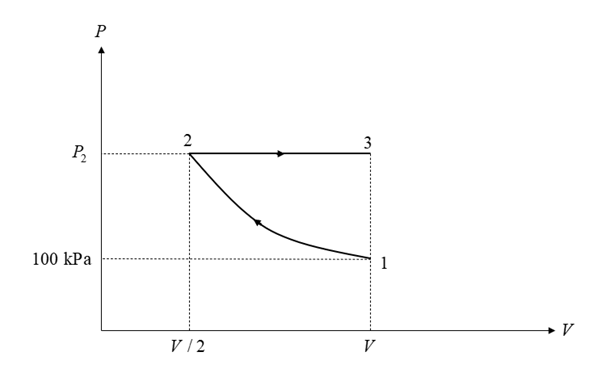
Given data:
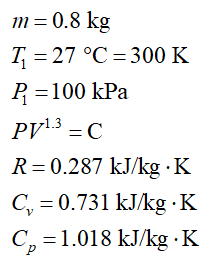
Step by stepSolved in 6 steps with 11 images

- pls answer the givenarrow_forwardEither solve all parts or leave it unsolved ... I vll upvotearrow_forward2. An ideal gas in a piston/cylinder system undergoes a process in which the specific volume is increased. In which process is the most work done for the same volume change? a. Isobaric process b. Isothermal process C. Isentropic process, k = 1.4 d. Polytropic process, n = 2 e. Cannot tell from this informationarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





