
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
The following diagrams represent three systems at equilibrium, all in the
same-size containers. (a) Without doing any calculations, rank the systems
in order of increasing Kc. (b) If the volume of the containers is 1.0 L and
each sphere represents 0.10 mol, calculate Kc for each system.
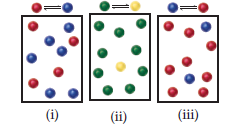
Transcribed Image Text:(i)
(ii)
(iii)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At 200 degrees C, Kc = 1.4*10^-10 for the reaction N2O (g) + NO2 (g) <--> 3NO(g) a) if 300 ml of NO measured at 800 torr and 25 degrees C is placed in a 4.00 L container, what will be the N2O and NO molar concentrations at equilibrium b) What will be the total pressure of the mixture (in atm) at equilibrium at 25 degrees Carrow_forwardWRITE THE EQUILIBRIUM EXPRESSIONS FOR EACH OF THE FOLLOWING: (A) CO(G) + 2H2(G) <- -> CH3OH(G) (B) 2NO2 (G) <- -> 2NO(G) + O2(G) (C) P4 (G) + 6 BR2 (G) <- -> 4 PBR3(G)arrow_forwards ppe (p) the eats of reaction. heactants 2. Write the equilibrium expression for each of the following reactions (a) 2HB1(g) → H2(g) + Br2(g) (b) CH4(g) + C2(g) → CH;Cl(g) + HCl(g)arrow_forward
- Need helparrow_forward17.101 The molecular scenes below depict the reaction Y at four different times, out of sequence, as it reaches equilibrium. Each sphere (Y is red and Z is green) represents 0.025 mol, and the volume is 0.40 L. (a) Which scene(s) represent(s) equilibrium? (b) List the scenes in the correct sequence. (c) Calculate K.. Aarrow_forwardFor the reaction N,0,(g) = 2NO,(g) Kc = 4.66 × 10-3 at 25°C . 2.50 g N,04 and 0.190 g NO, are introduced into a 2.00-L reaction vessel. After equilibrium is achieved, what is the concentration of NO,?arrow_forward
- Consider the reaction: A B The equilibrium constant for this reaction is Kc = 10. Which of the following diagrams best represents this reaction at equilibrium? Hint: grey spheres = A green spheres = B Od O c Ob O a (a) (b) O (d)arrow_forwardConsider the reaction, 2AB2(g) = A2(g) + 2B2(g), Kc = 0.263 at 25°C. The reaction begins with an unknown concentration of AB2 in the reaction flask. At equilibrium, the concentration of AB2 is found to be 0.00545 M. a) Complete the ICEE table below, b) Write the equilibrium expression and equation for this reaction (pay close attention to coefficients!), c) Calculate the equilibrium concentration of both products, and d) Calculate the initial concentration of AB2. You should confirm that your equilibrium concentrations will give the correct equilibrium constant when plugged into the expression. ICEE table I C E 2 AB₂(g) A₂(g) + 2 B₂(g)arrow_forward3. For the following reaction at equilibrium, 2BrNO(g) → 2NO(g) + Br2(g), what will happen if the volume of the container is decreased? (a) The reaction remains unchanged (b) The reaction will shift to the left (c) The reaction will shift to the rightarrow_forward
- Use the References to access important values if needed for this question. The equilibrium constant for the following reaction is 1.20×10-2 at 500K. PCI5(g) PCI3(g) + Cl½(g) If an equilibrium mixture of the three gases at 500K contains 3.57x10-2 M PCI,(g) and 2.23×102 M PCI3, what is the equilibrium concentration of Cl,? M Submit Answer Retry Entire Group 9 more group attempts remaining edarrow_forward(a) Determine whether the value of K of an equilibrium system will be affected or, unaffected by the following changes: Changes Value of K (affected or unaffected) (O Decreasing the temperature (1) Increasing the pressure of the system () Decreasing the concentration of reactant (b) The following graph represents the amount of CO formed at two different temperatures, T; and T. The reaction as shown below: C() + H,O(g) = co(g) + H(g) T = 385 K [co) T = 335 K time Based on the graph, determine whether the forward reaction is endothermic or exothermic. Predict if the concentration of H:0 gas increases, decreases or remains the same if the temperature is decreased to 298 K. Explain. A mixture of 0.350 M of H:0, 0.125 M of CO and 0.125 M of H: was prepared and allowed to reach equilibrium in a 1.00 L flask. The equilibrium concentration of H;0 is 0.182 M. Calculate the Ke of the reaction at constant temperature. (H)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY