
(a)
Reversible process:
A process is said to be a reversible process if it takes place in a manner that it can be reversed following the same path as that of the forward process without leaving any change in the system and surrounding.
Also, in the reversible process, the change in entropy will be zero. i.e.,

Irreversible process:
A process is called irreversible if the system and all parts of its surroundings cannot be exactly reversed back following the same path as that of the forward process without leaving any change in the system and surrounding.
Also, for the irreversible process,

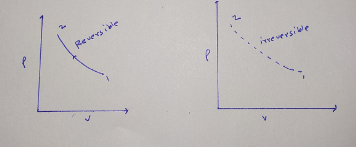
The thermodynamic graph for the reversible process is represented as solid line, while the irreversible process is represented as dotted line. As shown in the following diagram.
Step by stepSolved in 5 steps with 6 images

- Explain why it is impossivle for any process to be more than 100% efficient. Give an example of a process that is not very effecient and explain where it 'loses' energy.arrow_forward20 please need help, keep in mind the 7 basic units and watch the sig figs thank you so much.arrow_forwardWhat is entropy of a system? How can second law of thermodynamics be stated in terms of entropy?arrow_forward