
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Those go together
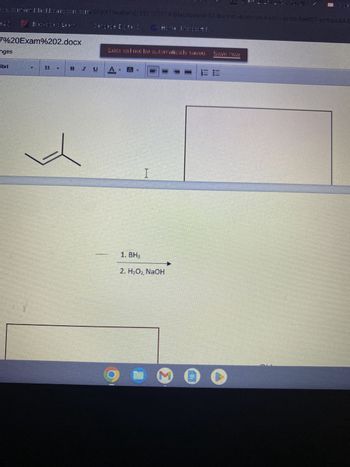
Transcribed Image Text:es comem blackbeancson.com/5fdcf19eabed/35110707X-Blackboard-S3-Bucket-leam-us-east-1-prod-fleet 01-xy thos&X-E
Home Marabot 3
385555
nges
Blacecard Learn
7%20Exam%202.docx
sage Digital D
Edits will not be automatically saved Save now
B ZU A- A
I
1. BH₂
2. H₂O₂, NaOH
000
0:20
LE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A saturated solution is a a. solution that can't dissolve additional quantities of solute. b. heterogeneous mixture. O . diluted solution. d. solution that can dissolve additional quantities of solute.arrow_forwardA solution of potassium chloride in water boils at a certain temperature. What could be done to raise the boiling point of this solution?arrow_forward3. What volume of 200M of CaCl2 stock solution would you use to make .50L of.300M CaCl2?arrow_forward
- 10A Complete the figure below. Br NaCN DMSO B A NH₂ C 1. Br₂, NaOH 2. H₂O/H* TT. H₂SO4arrow_forwardCalculate grams of sucrose that must be added to 206.8 grams of water to prepare a 18.32 % by mass solution. a. 46.4 b. 139 c. 92.8 d. 0.0969 e. 23.2arrow_forward1. Write balanced net ionic equations to explain the observations in the options assigned by your instructor. (All reactions are oxidation-reduction.) See the protocol on page 192. a) Option E Cues b) Option Farrow_forward
- e. The average human skin cell f. The length of the tip of your thumb (measured from first knuckle to the edge of your nail) 2. Consider two molecules, X and Y. a. X is able to dissolve in water. What does this imply about the favorability of interactions between molecules of X versus interactions between X and water? b. Y is unable to dissolve in water. What does this imply about the favorability of interactions between molecules of Y versus interactions between Y and water? Draw what happens to Y in water. 1arrow_forward#1arrow_forwardPlease helparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY