
Concept explainers
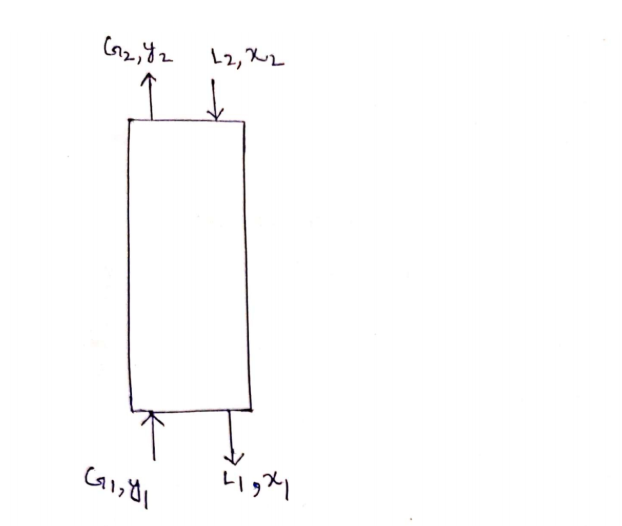
Hydrogen sulfide (H2S) gas in petroleum refinery is to be removed from a 1387kg/h gas mixture consist of 16 % w.w H2S into air, using counter current absorption column.
Water is used as solvent in the absorption column. The water flow rate is 3 times the gas mixture flow rate.
The air mole percent at the top of the column is 97.5%.
The air consists of 21.9% oxygen and 78.1% nitrogen.
Calculate;
4. The mole fraction of H2S in the liquid stream leaving the bottom of the tower?
Note; Don’t round numbers; Minimum 4 decimals (0.0000)
Given
A) The air consists of 21.9% oxygen and 78.1% nitrogen.
B) The atomic weight of;
1. The hydrogen atom is 1
2. The Oxygen atom is 16
3. The sulfur atom is 32
4. 1The nitrogen atom is 14

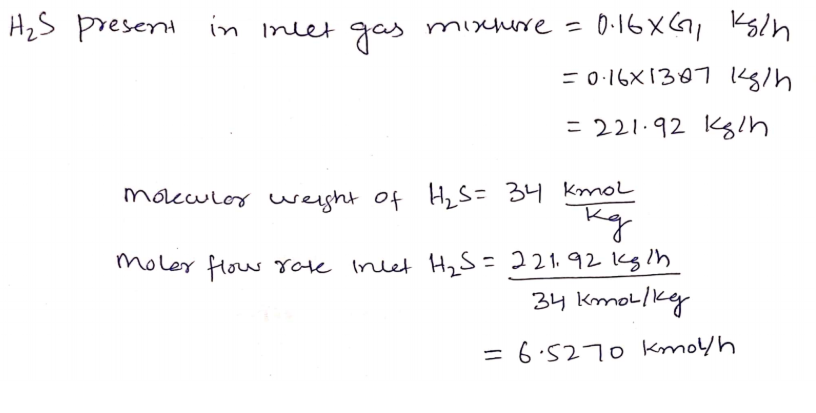
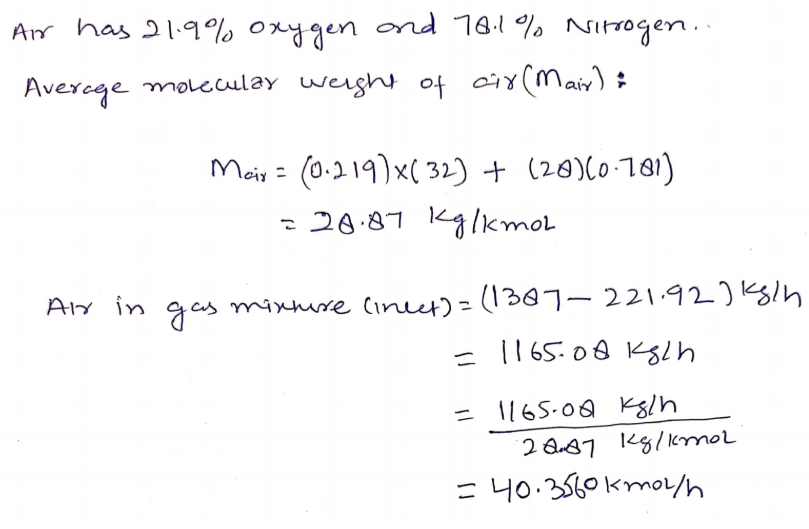
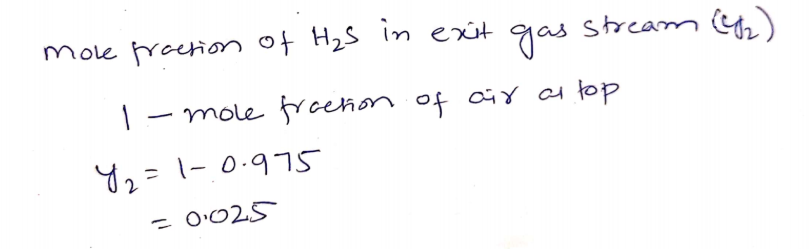

Step by stepSolved in 8 steps with 10 images

- Hydrogen sulfide (H2S) gas in petroleum refinery is to be removed from a 1387kg/h gas mixture consist of 16 % w.w H2S into air, using counter current absorption column. Water is used as solvent in the absorption column. The water flow rate is 3 times the gas mixture flow rate. The air mole percent at the top of the column is 97.5%. The air consists of 21.9% oxygen and 78.1% nitrogen. Calculate; 2. The mole flow arte and mole fraction of H2S in the gas stream leaving at the top of the tower? Note; Don’t round numbers; Minimum 4 decimals (0.0000) Given A) The air consists of 21.9% oxygen and 78.1% nitrogen. B) The atomic weight of; 1. The hydrogen atom is 1 2. The Oxygen atom is 16 3. The sulfur atom is 32 4. 1The nitrogen atom is 14arrow_forwardHydrogen sulfide (H2S) gas in petroleum refinery is to be removed from a 1387kg/h gas mixture consist of 16 % w.w H2S into air, using counter current absorption column. Water is used as solvent in the absorption column. The water flow rate is 3 times the gas mixture flow rate. The air mole percent at the top of the column is 97.5%. The air consists of 21.9% oxygen and 78.1% nitrogen. Calculate; 3. To calculate the quantity and percent of H2S absorbed in the column? Note; Don’t round numbers; Minimum 4 decimals (0.0000) Given A) The air consists of 21.9% oxygen and 78.1% nitrogen. B) The atomic weight of; 1. The hydrogen atom is 1 2. The Oxygen atom is 16 3. The sulfur atom is 32 4. 1The nitrogen atom is 14arrow_forward______ marrow_forward
- A petroleum gas mixture containing 65% propane, 25% of propylene and 10% of butane (mole percent) is burned with with 40% excess air. All the butane and propylene are consumed and 90% of the propane. There is no CO in the outlet gas. Calculate the composition of the flue gases on a dry basis, assuming that the moisture content of the air is negligiblearrow_forwardHydrogen sulfide (H2S) gas in petroleum refinery is to be removed from a 984 kg/h gas mixture consist of 25 % w.w H2S into air, using counter current absorption column. Water is used as solvent in the absorption column. The water flow rate is 1.81 times the gas mixture flow rate. The air mole percent at the top of the column is 96.5%. The air consists of 21.9% oxygen and 78.1% nitrogen. Calculate; To H2S molar flow rate and mole fraction entered the bottom of the column? Note; Don’t round numbers; Minimum 4 decimals (0.0000) Given A) The air consists of 21.9% oxygen and 78.1% nitrogen. B) The atomic weight of; 1. The hydrogen atom is 1 2. The Oxygen atom is 16 3. The sulfur atom is 32 4. 1The nitrogen atom is 14arrow_forwardYou now work for the new biogas company ‘WhiffGen’ who are scaling up a process to take waste bio materials and produce solid fuel pellets for biomass boilers. As a part of the feedstock treatment, chloride is removed as this would cause corrosion and possible dioxin generation in the biomass boilers. This chloride removal process produces a waste gas stream containing hydrogen chloride acid gas at 7% by volume in dry air.The packed column is to treat 175 m3 s-1 of the hydrogen chloride gas stream to a concentration of 0.3% by volume. The water used is high purity and is to have a concentration of 5% by weight after being passed through the column. Draw a simple block diagram of the column with the input and output streams labelled and calculate the volumetric flow rate of gas leaving the column.arrow_forward
- A gas mixture consisting of 80% ethane and 20% oxygen is burned in an engine with200% excess air. 80% of the ethane goes to CO2, 10% to CO and 10% remains unburned. Calculate the composition of the exhaust gases on(a) a wet basis(b) a dry basis pls indicate which is a wet and dry basis in the solution.arrow_forwardHydrogen sulfide (H2S) gas in petroleum refinery is to be removed from a 984 kg/h gas mixture consist of 25 % w.w H2S into air, using counter current absorption column. Water is used as solvent in the absorption column. The water flow rate is 1.81 times the gas mixture flow rate. The air mole percent at the top of the column is 96.5%. The air consists of 21.9% oxygen and 78.1% nitrogen. Calculate; The mole flow rate and mole fraction of H2S in the gas stream leaving at the top of the tower? Note; Don’t round numbers; Minimum 4 decimals (0.0000) Given A) The air consists of 21.9% oxygen and 78.1% nitrogen. B) The atomic weight of; 1. The hydrogen atom is 1 2. The Oxygen atom is 16 3. The sulfur atom is 32 4. 1The nitrogen atom is 14arrow_forwardSEPERATIONS Absorption: Contaminant in a Gas Effluent We are going to absorb 90% of a contaminant in a gas stream containing 15.0 mole of the contaminant by absorntion into a water phase. The total inlet gas flow to the absorption column is 100.0 kgmole/hr and the total water flow is 200 kgmole/hr. Inlet water is NOT pure but contains 0.5% of the contaminant. I he process operates isothermally at 300k and a pressure of 1 atm. The equilibrium for this system is effectively characterized by either a Henry's Law relationship or Raoult's Law (using partial pressure), a general equilibrium relationship that can be used is ya=1.60*xa. plot the operating line and determine the minimum number of stages (graphically) for the equilibrium relationship provided. Confirm the results of part (a) using the analytical Kremser equation.arrow_forward
- To make an instant tomato soup, a company makes soup powder from liquid step by a two-step process: concentrating the soup by removing water using a membrane to reach a concentration of 45% water, followed by a spray drying process which leads to a final moisture content of 3%. If the initial liquid tomato soup contains 9.0% solids and fat and enters the membrane separator at 4000 kg/hr, calculate: The flow-rate of the concentrated soup after membrane separation: kg/hr The flow-rate of water removed by the spray drying process (the second stage): kg/hrarrow_forwarda double effect evaporator is fed by 31896 lbm/hr of 9% potassium chloride solution. At the end of evaporation a 41% KCl solution is obtained. The water evaporated in the second effect is 1.5 times the water evaporated in the first effect. Determine:a) The amount of 41% concentrated solutionb) The amount of water evaporated in the first and second effectsc) The amount of solution fed into the second effect, as well as its compositionarrow_forwardA 0.001 in. BCC iron foil is used to sepa- rate a high hydrogen content gas from a low hydrogen content gas at 650°C. 5 × 10% H atoms/cm³ are in equilibrium on one side of the foil, and 2 × 10³ H atoms/cm³ are in equilibrium on the other side. Determine (a) the concentration gradient of hydro- gen; and (b) the flux of hydrogen through the foil.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





