
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
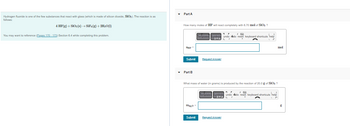
Transcribed Image Text:Hydrogen fluoride is one of the few substances that react with glass (which is made of silicon dioxide, SiO₂). The reaction is as
follows:
4 HF(g) + SiO₂ (s) → SiF4 (g) + 2H₂O(1)
You may want to reference (Pages 170-173) Section 6.4 while completing this problem.
Part A
How many moles of HF will react completely with 6.70 mol of SiO₂?
Symbols undo redo reset keyboard shortcuts help
A
THE
Submit
Part B
mg,0
Templates
Submit
Request Answer
What mass of water (in grams) is produced by the reaction of 20.0 g of SiO₂ ?
Template: ymbols undo do reset keyboard shortcuts help
PAS
Request Answer
mol
A
g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- suppose a student reacts 1.57 g Ca with O2 gas. In his/her calculations however S/he mistakenly calculate the theoretical yield with 1.97 g Ca. Will the theoretical yield of CaO be too high, too low, or unaffected?arrow_forwardFor the reaction N, + 3H, 2NH, what is the Mole ratio of nitrogen to hydrogen? t. Mole ratio of hydrogen to ammonia?arrow_forwardConsider the balanced reaction of magnesium and oxygen. 2Mg+O2⟶2MgO What mass, in grams, of MgO can be produced from 1.61 g of Mg and 2.75 g of O2?arrow_forward
- Payalarrow_forwardCryolite, Na3AlF6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. Balance the equation. 1.) Balance the equation - AlO3(s)+NaOH(l)+HF(g)-->Na3AlF6+H2O(g) 2.)If 16.4 kilograms of Al2O3(s), 60.4 kilograms of NaOH(l), and 60.4 kilograms of HF(g) react completely, how many kilograms of cryolite will be produced? 3.)Which reactants will be in excess, (Al2O3, NaOH, or HF) 4.)What is the total mass of the excess reactants left over after the reaction is complete in KG?arrow_forward1. (Balance the equation) H,SO+NaOH>H,SO+H,Oarrow_forward
- Write the chemical formula and indicate the physical state of the products. Example: PbC12 (s) + H2O (I). carbonic acid (aq) + potassium hydroxide (aq) ==>arrow_forwardA Group 2 metal with an electron configuration of [Ne]3s2 combines with fluorine (F2) to produce a compound. In g/mol, what is the molar mass of the product?arrow_forwardWhat mass (in grams) of oxygen is required to make 27.5 g calcium carbonatearrow_forward
- What mass of HF would be produced from 4.6L of f2 gas warrow_forwardA young man recently passed away from unknown causes. The newly hired forensic officer, Citlali, recalled her time in chemistry as she performed combustion analysis and found the young man had high amounts of a compound containing C, H and O. Combustion of the 9.002 g substance produces 0.450 moles of CO₂, and 0.201 moles of H₂O (Hint Law of conservation). Determine molecular formula of the unknown compound if the molar mass of the compound is between 150 and 210 g/mol.arrow_forwardConsider the balanced reaction of magnesium and oxygen. 2Mg+O2⟶2MgO2Mg+OX2⟶2MgO What mass, in grams, of MgO can be produced from 1.72 g of Mg and 2.39 g of O2?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY