
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How would you interpret this IR spectra of fluorene? What I mean by this is which peaks let you know its fluorene and which peaks show impurities if any such as 9-fluorenone since this lab required a separation of fluorene and 9-fluorenone. Other impurities could be solid alumina and sand and the solvents of pet. ether and dichlormethane.
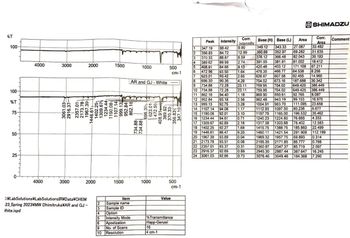
Transcribed Image Text:%T
100
100-
%T
75-
50-
25-
0
4000
4000
3000
3061.03-
2916.37-
3000
2000
2000
LabSolutions Lab SolutionsIR¥Data CHEM
23 Spring 2023 MW Dhimitruka¥AR and GJ -
Vhite.ispd
2
3
1500
Item
Sample name
Sample ID
Option
5 Intensity Mode
6
4
1500
Apodization
No. of Scans
9
10 Resolution
1000
AR and GJ - White
734.881
734.88
596.30
623.01
472.56
1000
408.91
16
4 cm-1
500
cm-1
389.62
370.33
347.19
356.83
500
cm-1
Value
%Transmittance
Happ-Genzel
696.30
1090.30
Corr.
Intensity
9.90
12.99
6.59
2.74
9.13
1.64
2.65
4.29
14.29
Peak
1
88.42
347.19
2 356.83 84.72
3 370.33 88.87
4 389.62 89.99
408.91
472.56
69.99
84.68
5
6
1472.00
7
623.01
8
0
9
734.88
734.00
72.28
123 11
12.20
10
72.28
23.11
23.11
1.18
2.56
3.28
117
1.17
13
14
15
16
40
3.10
74
734.88
11
862.18
94.40
12 952.84 93.18
999.13 92.75
1107.14 94.08
1190.08 92.01
1234.44 94.61 0.71
17 1309.67 92.89 2.18
18 1402.25 92.27 1.68
19 1446.61 86.47 9.20
20 1967.39 93.89 0.04
21 2173.78 93.51 0.08
22 2357.01 93.37 0.31
23 2916.37 92.69 0.89
24 3061.03
92.66
0.73
Intensity
93.50
93.42
09:44
90.36
SHIMADZU
Corr.
Area
Area
33.492
Base (H) Base (L)
349.12
27.067
343.33
360.69 352.97 69.282 51.635
376.12 366.48 82.043 35.193
391.55 381.91 81.002 18.412
420.48 403.12 171.108 67.211
466.77 64.538
478.35
8.256
626 87 607.58 92.455 14.960
1704.02
187.688
673.16
30.342
759.95 704.02 649.425 386.449
1704.02
759.95
649.425 386.449
869.90 850.61 92.765 6.067
8.087
962.48
943 19 99.153 16.970
1004.91
1112.93
4407 70
10.970
983.70
1097.50
111.095
80.236
1165.00 196.532
23.658
6.677
35.462
4.333
1197.79
1240.23 1224.80 76.665
1317.38 1303.88 78.402 12.583
1415.75 1388.75 185.860
1460.11 1421.54 291.909
22.499
112.199
0.314
0.788
1969.32 1957.75 69.893
2185.35 2171.85 85.777
2360.87 2347.37 85.719 2.097
2945.30 2887.44 387.647 16.245
3076.46 3049.46 184.368 7.290
Comment
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need help with making an ir spectrum Analysis for Cyclohexanol that includes in a table: Ir absorbtion bands cm^- and functional group in the fuctional group i have to connecet it to a specific compung group I thinkarrow_forwardIf you ran an unknown sample on a TLC using 3-Methyl-Pentane as eluent and observed only one spot with a Rf value -0.05, is it safe to assume that it is a pure compound? If not, how can you check it out using TLC?arrow_forwardIf the analysis by IR spectroscopy showed that 4-t-butylcyclohexene contains 4-t-butylcyclohexanol, what quantitative separation technique would you use to get to pure cycloalkene. EXPLAIN ALL DETAILS OF THE PROCESS TO FOLLOW until the pure product is weighed.arrow_forward
- References] The molecular ion in the mass spectrum of 3-methyl-2-pentene appears at m/z 84. Propose a structural formula for the prominent peak at m/z 29. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. • Do not use the square brackets tool in your answer. C P opy aste CHarrow_forwardMatch the molecules from the drawings that is most likely to have produced each of the three Mass Spectrum. Then identify the likely breakdowns that yield each major peak.arrow_forwardAssign the IR spectra given in Questions 1 - 3 to their corresponding structures from the ones below. The number in parentheses next to each possible answer corresponds to the structure's Chemical Abstracts Services (CAS) number. 1-bromobutane (109-65-9) 1-cyclopropylethan-1-one (765-43-5) 1-ethyl-3-methylbenzene (620-14-4) 1,2-dimethoxynenzene (91-16-7) 2,3-dimethylbutan-2-ol (594-60-5) 3-methylbutan-1-ol (123-51-3) 3,3-dimethylbutan-2-one (75-97-8) 4-benzylpiperidine (31252-42-3) 4-methoxybenzaldehyde (123-11-5) acetic anhydride (108-24-7) acetonitrile (75-05-8) acetyl chloride (75-36-5) anisole (100-66-3) benzaldehyde (100-52-7) benzoic acid (65-85-0) benzophenone (119-61-9) benzyl acetate (140-11-4) bromobenzene (108-86-1) cyclohex-2-en-1-one (930-68-7) cyclohexanone (108-94-1) cyclopent-2-en-1-one (930-30-3) diethylamine (109-89-7) dimethyl malonate (108-59-8) ethyl 2-cyanoacetate (105-56-6) ethynylbenzene (536-74-3) heptanoic acid…arrow_forward
- Please don't provide handwriting solutionarrow_forward5. The proxluct of the reaction below gives the IR spectrum shown. Although you are unfamiliar with the rcaction, usc your knowledge of IR spectroscopy to predict a likely product. (Note: the number of carbon atoms in the product is the same as in the starting material.) Page 4 OH Al(OiPr)3 acetone no 4000 c hle belveen sall rlate cas. e Ged LIFEarrow_forwardFor each structure, list the bond stretches that will produce separate bands in the IR between 4000 and 2000 cm-1 and give approximate frequency.arrow_forward
- Assign the IR spectra given in Questions 1 - 3 to their corresponding structures from the ones below. The number in parentheses next to each possible answer corresponds to the structure's Chemical Abstracts Services (CAS) number. 1-bromobutane (109-65-9) 1-cyclopropylethan-1-one (765-43-5) 1-ethyl-3-methylbenzene (620-14-4) 1,2-dimethoxynenzene (91-16-7) 2,3-dimethylbutan-2-ol (594-60-5) 3-methylbutan-1-ol (123-51-3) 3,3-dimethylbutan-2-one (75-97-8) 4-benzylpiperidine (31252-42-3) 4-methoxybenzaldehyde (123-11-5) acetic anhydride (108-24-7) acetonitrile (75-05-8) acetyl chloride (75-36-5) anisole (100-66-3) benzaldehyde (100-52-7) benzoic acid (65-85-0) benzophenone (119-61-9) benzyl acetate (140-11-4) bromobenzene (108-86-1) cyclohex-2-en-1-one (930-68-7) cyclohexanone (108-94-1) cyclopent-2-en-1-one (930-30-3) diethylamine (109-89-7) dimethyl malonate (108-59-8) ethyl 2-cyanoacetate (105-56-6) ethynylbenzene (536-74-3) heptanoic…arrow_forwardSay you’ve just ran your unknown sample on the uv/vis and realized theabsorption is greater than the largest absorption of your standard curve. This is bad from a quantitative standpoint, as we can’t compare our standard curve with our data. What would you need to do to fix the problem? (Hint: there are two possible answers for this.)arrow_forwardAnalyze the following GCMS spectra. In your analysis of each GC-MS spectrum, include: a) The structure of the compound b) The molecular ion (M*) and its corresponding m/z value (if shown in the spectrum) c) The base peak and its corresponding m/z value d) Provide the fragmentation pattern of the molecule. Be sure to clearly indicate the cations and/or radical fragments and their corresponding m/z value from the spectrum. Compound Name: 2-Hexanone 120 43 100 80 57 20 100 85 27 29 71 59 49 50 51 52 53 s4 5 101 0 31 160 62 63 65 67 69 83 14 10 20 30 40 50 60 70 80 90 100 m/z Relative Intensityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY