
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
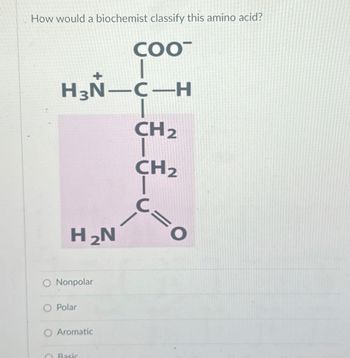
Transcribed Image Text:How would a biochemist classify this amino acid?
COOT
H3N-C-H
H2N
O Nonpolar
Polar
Aromatic
Basic
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Which of these molecules is not a natural amino acid?arrow_forwardLook at the amino acids shown below. Their side chains are highlighted, Which amino acids have polar side chains? *** H,N-Ç-COOH H,N- C-COOH COOH H,N-C-COOH CH2 CH, H. H-C-CH, NH3 ČH, H. H. OH Вarrow_forward41 Select all functional groups present in the amino acid shown. (Hint: There will be more than one functional group present.) alcohol aldehyde alkene alkyne amine amide aromatic ring carboxylic acid ester ether ketone thiolarrow_forward
- Question 6 of 15 Submit Classify the amino acid depicted here in the form at physiological pH (~7.4). O: H₂N* A) acidic B) basic C O C) nonpolar D) neutral HU CH₂ O C JU +arrow_forwardWhat tertiary interaction would be expected between the following amino acids OH H,N-CH OH CH-CH3 CH2 ČH, O covalent bond O electrostatic attraction O hydrogen bonding O hydrophobic O no tertiary interaction expectedarrow_forwardThis amino acid would be more likely to bind to a receptor inside of the cell. COO- H3N-C-H CH2 CH2 CH2 CH2 *NH3 True O False This amino acid would be more likely to bind to a receptor inside of the cell. COO H3N-C-H H-C-CH3 CH2 CH3 O True O Falsearrow_forward
- Which of the following compounds is not an amino acid?arrow_forwardTwo of the amino acids have two chiral centers. Give the name and three-letter abbreviation of each. Enter your answers separated by a comma.Enter your answers separated by a comma.arrow_forwardH;N-C-C-OH Type in the name of the dry amino acid (all lower case, c HO H.N-C-C-OH -ОН CH2 OHarrow_forward
- Which amino acid is this? H₂N- hono CH-C-OH CH₂arrow_forwardwhich group determines the properties of amino acids, indicated by what number? explainarrow_forwardPart D Complete the sentences to explain the differences between tertiary and quaternary structures of proteins. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. three-dimensional shape two or more peptide subunits oxygen and disulfide bonds -R groups two-dimensional shape two or more polypeptide chains disulfide bonds and salt bridges -C(O)NH groups Submit Request Answer The tertiary structure is determined by the interactions of describes the of the protein In the quaternary structure, Reset Help These interactions, such as interact to form an active protein.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY