
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How much would be 20.Mg in lbs and how would I put the answer into sig figs and scientific notation?
Expert Solution
arrow_forward
Step 1
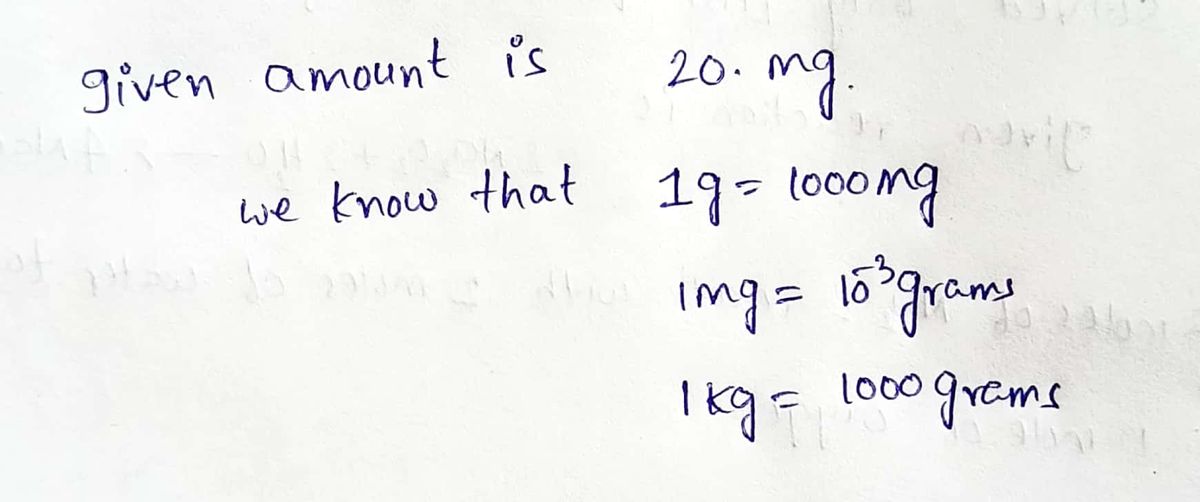
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What volume of water (ml) would a 51.4 g sample of pure gold displace? The density of gold is 19.3 g/cm,arrow_forwardconvert and use sig figs 2.75kg to oz.arrow_forwardSuppose the densities of two metals are so close together that density is difficult to use to be able to distinguish them within the margin of error (a good example would be zinc and tin). What other physical properties could you use to determine which metal was which? Explain your reasoning thoroughly.arrow_forward
- A pill that is 91.5% aspirin is found to contain 59.99 mg of aspirin. What is the mass of the pill in milligrams?arrow_forwardA chemist adds 800.0 mL of a 50.0 g/dL calcium bromide (CaBr,) solution to a flask. Calculate the mass in kilograms of calcium bromide the chemist has added to the flask. Be sure your answer has the correct number of significant digits. O kgarrow_forwardPlease helparrow_forward
- A student was given Mg ribbon that's 189 mg and 98.7 mm long. If the ribbon is uniform, what length of Mg ribbon would be needed in order to have a measured volume of 44.4 mL of H2?arrow_forwardThe center on the basketball team is 6 ft. 10 in. tall. How tall is the player in millimeters? Your final answer should have 3 sig figs.arrow_forward1.) Convert 21.37 mL to L. Final answer into scientific notation 2.) How many years in 1 billion (1x10⁹) seconds?arrow_forward
- A bar of gold measures 0.127 m x 0.0483 m x 0.0155 m. How many gallons of water have the same mass as this bar? Number Unitsarrow_forwardarch E C $ 4 101 R F V 15 % Convert 3.19 × 10-5 5 ADD FACTOR * ( ) 319 3.19 x 104 3.19 x 10-12 3.19 x 10-14 0.0319 T 0.01 G O 16 40 6 Y 10 0.001 H & 7 m to nanometers Use only the metric system. BN U 10-⁰ J [+ 8 M 1000 # fo 3.19 10⁰ 144 ( O 9 ANSWER 3.19 x 10- 10⁰ fo JI K 3.19 x 10" O ▶11 ) O L 3.19 x 10⁰ f11 PI P : 31.9 ; RESET 10⁰ S 112 { ? + [ 44 0.1 = 1 $ 1 ins prt sc } ← backspace ] delete ( pause home. num lock enter T shiarrow_forward(a) 8.160 m = ________ cm (b) 781 mL = ________ L (c) 4.18 kg = ________ g (d) 27.8 m = ________ km show your work please 2. The expressions below all mean the same thing when it comes to unit conversions. 1 inch = 2.54 cm 2.54 cm/1inch 1inch/2.54 cm Question 2 options: True Falsearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY