
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please provide the complete solution. Thank you!
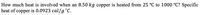
Transcribed Image Text:How much heat is involved when an 8.50 kg copper is heated from 25 °C to 1000 °C? Specific
heat of copper is 0.0923 cal/g °C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Six 50.0 ml volumetric flask 1 through 6 are labeled. 10.00 ml of 2.00 x 10-1 M Fe(NO3)3 solution was then pipet into each volumetric flask. A 1.00,2.00,3.00,4.00 and 5.00ml of 2.00 x 10-3M NaSCN solution was then pipetted to flask 2 through 6 respectively. A sufficient amount of 0.10 M nitric acid was then added to each flask to make each a total volume of 50.00ml. Hint M1V1=M2V2 a)what would be the concentration of Fe(NO3)3 in 0.10M HNO3? b)what would be the concentration of NaSCN in 0.10M HNO3?arrow_forwardAn impure sample of 250 mg of adipic acid was recrystallized from 2.0 mL of boiling water. The solution was cooled to room temperature slowly and further cooled in an ice bath. What is the maximum amount of recoverable adipic acid at 0 oC? Solubility of adipic acid at 0 oC is 0.3 g/ 100 mL Group of answer choices 6 mg 249 mg 244 mg 250 mgarrow_forward43.27 mL of 0.5033 M NaOH are required to neutralize 29.16 mL of a H2SO4 solution.What is the molarity of the H2SO4 solution? H2SO4 + 2 NaOH ® Na2SO4 + 2 H2Oarrow_forward
- How would you prepare 2 L of 1 M MgCl^2 from a 5 M MgCl^2 stock solution?arrow_forwardHow many liters of softened water, containing a sodium concentration of 4 * 10 ^ - 2 % sodium by mass, have to be consumed to exceed the FDA recommendation ? (Assume a water density of 1g / m * L ) Express your answer using two significant figures.arrow_forwardcalculate the volume of cyclohexanonrequired tomakee 25 mL of 40ppm cyclohexanon solutionarrow_forward
- Write a balanced chemical equation to represent the reaction between bleach and hydrochloric acid?arrow_forwardUse the virtual lab at Chem Collective http://chemcollective.org/activities/vlab/3 Choose 1 of the following solutions to make: 1.75 M HNO3 1.3 M HClO4 2.4 M H3PO4 It is up to you how much of each solution you choose to make but I would recommend no less than 100 mL. Write down and submit the following for EACH of the 2 stock solutions you made: How much in mL of the stock solution did you use? How much water in mL did you add? What is the resulting molarity?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY