
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Completed 13 out of 36
Submit All
KA
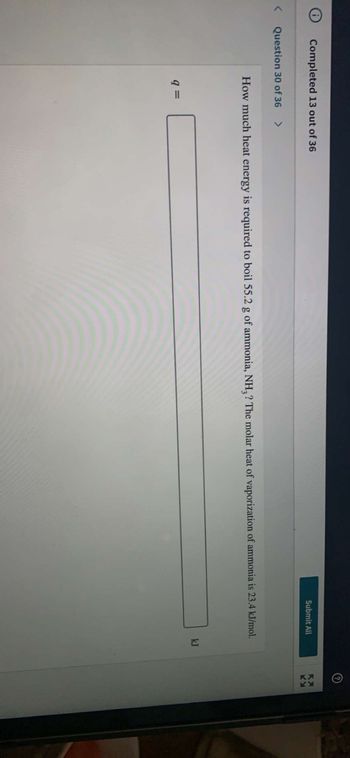
How much heat energy is required to boil 55.2 g of ammonia, NH3? The molar heat of vaporization of ammonia is 23.4 kJ/mol.
kJ
9 =
< Question 30 of 36 >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How much heat is liberated at constant pressure when 1.41 g of potassium metal reacts with 6.52 mL of liquid iodine monochloride ( d = 3.24 g/mL)? 2K(s) + ICl(l) → KCl(s) + KI(s) ΔH° = –740.71 kJ/mol a. 1.23 × 10^2 kJ b. 7.41 × 10^2 kJ c. 2.22 × 10^3 kJ d. 9.64 × 10^1 kJ e. 1.34 × 10^1 kJarrow_forwardIf the heat of combustion for a mole of methane (CH) is -2,200 kJ, how many grams of methane must be burned to heat 455 g of ice at 0°C to water at 88°C?arrow_forward7. An old "trick" for cooling down a hot beverage quickly is to immerse a cold spoon into the liquid. A silver spoon weighing 3.20 oz and at a temperature of 20°C is placed in a cup of hot coffee (8.00 oz) at 70°C. What will be the resulting liquid temperature? Assume that specific heat of coffee is 4.18 J g-1 °C-1 and that of silver is 0.234 J g-¹ °C-1. Comment on the effectiveness of this method of cooling a hot drink. 1 g = 0.0352 ozarrow_forward
- GRB (S) AH = -325.0 kJ Using the following balanced equation: G2 (g) + 6R (s) → 2GR3 (s) AH = -325.0 kJ Atomic masses: • G: 15.00 g/mol R. 25.00 g/mol Calculate how many grams of solid R are required to produce 975.0 kJ of heat. Uparrow_forward6. Suppose 1.0 x 102 grams of ice absorbs 1255 J of heat, reaching a final temperature of -2.0°C. What was the initial temperature change of the ice? The specific heat capacity of H20(s) = 2.1 J/(g.°C).arrow_forward29. (a) (b) (c) What is the definition of the specific heat of water? the amount of heat required to melt one mole of a substance at the melting point. the amount of heat required to vaporize one mole of a Kryptonite at the boiling point. the amount of heat required to raise the temperature of 1 gram of water by 1°C.arrow_forward
- Calculate the heat necessary to increase temperature of a) 100. g of water b) 2.00 mol H2O(l) from 20 ℃ to 100 ℃.arrow_forward25.7 g of ice at 0 C is converted to liquid water at the same temperature. How much heat is absorbed?arrow_forwardCalculate the heat change in joules for vaporization of 9.00 g of water at 100 ∘C.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY