
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
9
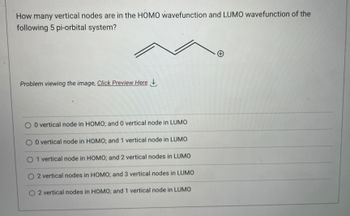
Transcribed Image Text:How many vertical nodes are in the HOMO wavefunction and LUMO wavefunction of the
following 5 pi-orbital system?
Problem viewing the image. Click Preview Here
O 0 vertical node in HOMO; and 0 vertical node in LUMO
O 0 vertical node in HOMO; and 1 vertical node in LUMO
O 1 vertical node in HOMO; and 2 vertical nodes in LUMO
O2 vertical nodes in HOMO; and 3 vertical nodes in LUMO
O 2 vertical nodes in HOMO; and 1 vertical node in LUMO
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many nodes are there in the p LUMO wavefunction of 1,3,5-hexatriene? Answer with ONLY an integer.arrow_forwardPlease answer correctly by yourself. Find the most probable value of radius for 3d orbital.arrow_forwardProblem What values of the angular momentum (l ) and magnetic (ml) quantum numbers are allowed for a principal quantum number (n) of 3? How many orbitals are allowed?Plan We determine allowable quantum numbers with the rules from the text: l values are integers from 0 to n - 1, and ml values are integers from +l to 0 to +l. One ml value is assigned to each orbital, so the number of ml values gives the number of orbitals.arrow_forward
- a) Write down a relation giving the number of electrons occupying the energy states between the energy interval dɛ at ɛ.arrow_forwardPlease do it fastarrow_forwardWhat values of J may occur in the terms 3D, 4D, and 2G? How many states (distinguished by quantum number MJ will belong to each level? SHOW PROCEDURE CLEARLY AND EXPLICITLY. DO NOT SKIP ANY STEParrow_forward
- Q/ Calculate the wave number and Prequencyof the Rirst line of the Ballmer series ?arrow_forward3. example of 1) Linear operation , 2) hermitian operationarrow_forwarde. Notice that the center atom and the two corner atonms along a diagonal all touch. What is the length of the diagonal in terms of "r" the radius of the atom? Answer:arrow_forward
- Explain why a photon blur of a fast-moving fan blade is a better analogue to an electron cloud picture than your target picture.arrow_forwardSolve correctly please. (Gpt/ai wrong answer not allowed)arrow_forwardWhat are the relations of the excited singlet and triplet state to emit light energy? Please answer shorty at your own words. Answer should be to the point.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,
