
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
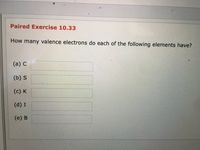
Transcribed Image Text:**Paired Exercise 10.33**
**Question:** How many valence electrons do each of the following elements have?
(a) C ________
(b) S ________
(c) K ________
(d) I ________
(e) B ________
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Arrange in order of increasing nonmetallic character. (a) the period 4 elements Ga, Ge, Ti (b) the Group 5A elements P, Bi, and Narrow_forward#9arrow_forward29) Predict the number of valence electrons for a Group 5A/15 element.(a) 2 (b) 3(c) 5 (d) 8(e) 1530) Which of the following groups has a predictable ionic charge of three positive?(a) Group 3A/13 (b) Group 3B/3(c) Group 5A/15 (d) Group 5B/5(e) Group 8A/1831) What is the electron configuration for a sulfide ion, S2-?(a) 1s22s22p63s23p2(b) 1s2 2s22p63s23p4(c) 1s22s22p63s23p6(d) 1s2 2s22p63s23p44s2(e) 1s22s22p63s23p64s232) The compound NH3is classified as which of the following?(a) ionic (b) polyatomic ionic(c) binary molecule (d) binary acid(e) oxyacid33) Aqueous H2SO4 is classified as which of the following?(a) ionic (b) polyatomic ionic(c) binary molecule (d) binary acid(e) oxyacid34) The NH4+ ion is classified as which of the following?(a) monoatomic cation (b) monoatomic anion(c) polyatomic cation (d) polyatomic anion(e) none of the above35) What is the chemical formula for the oxide ion?(a) O-(b) O2-(c) O2-(d) O22-(e) none of the above36) What is the chemical formula for the…arrow_forward
- Calculate the ratio between the radius of the radium nucleus and the radius of the oxygen nucleus. The atomic mass of the radium nucleus is A,, = 226 and the atomic mass of the oxygen nucleus is A, =16. (а) 1.5 (b) 3.0 (с) 2.4 (d) 6.0arrow_forward29) Predict the number of valence electrons for a Group 5A/15 element.(a) 2 (b) 3(c) 5 (d) 8(e) 15 please provide explanation step by step and explain and give the correct letterarrow_forward4. What is the atomic number? (a) 14 (b) 7 (c) 4 (d) 10arrow_forward
- a ) Eva l u a te t h e expre s s i o n s 2 x 1, 2 x (1 + 3),2 x(1 + 3 + 5), and 2 x (1 + 3 + 5 + 7). (b) How do the atomic numbers of the noble gases relate to the numbersfrom part (a)? (c) What topic discussed is thesource of the number “2” in the expressions in part (a)?arrow_forwardWhich of the following ions has the smallest size? (a) Cl- (b) F- (c) Al3+ (d) Na+arrow_forwardWrite the following from largest to smallest size. (a) Mg2+, Be2+, Mg (b) Cl−, Sc3+, K+, Ca2+arrow_forward
- The elements of the periodic . Table can be divided into main categories? (A) 3 (B) 5 (C) 4 (D) 2arrow_forward2. Draw the electron dot formula for the following elements. (a) C. (b) He (c) Alarrow_forwardDetermine the number of protons, neutrons, and electrons in the following isotopes that are used in medical diagnoses: (a) atomic number 9, mass number 18, charge of 1− (b) atomic number 43, mass number 99, charge of 7+ (c) atomic number 53, atomic mass number 131, charge of 1− (d) atomic number 81, atomic mass number 201, charge of 1+ (e) Name the elements in parts (a), (b), (c), and (d).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY