
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
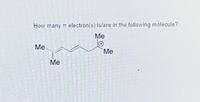
Transcribed Image Text:How many TT electron(s) is/are in the following molecule?
Me
Me
Me
Me
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many lone pairs of electrons are on the B atom in BCI3 (lone pair = 2 electrons not in a bond) « Previous Ne Not saved Subr rses/2921231/quizzes/642 311/take/questions/111655468 MacBook Air F2 DII F3 F4 F6 F9 %23 $ 4 & 6. 7 9. E R. T. Y H K C V * 00 F.arrow_forwardmolecule Molecule Total # Valence e Lewis Structure Electronic geometry (name & drawing) Molecular geometry (name & drawing) CH4 (methane) Hi :H Hいl:-H Tetrahedra) Tatrahedral NH3 (ammonia) Hu N-H Fetrahedral Hi puramidal H2O (water) O= 6 H=Ie) H:0:H te trahedral Tetrahedral 8e CO2 C=4 O=2(6) (carbon dioxide) 0::C::0: O=C=0 16e C=4 CH2O (formaldehyde) O=6 H: C: H H-C-H 12 e Ang onal planar trigonal planar :2:エarrow_forwardMark the following statements as true or false. A molecule with very polar bonds can be nonpolar. blank The electrons in a polar bond are found nearer to the more electronegative element. blank Fluorine is very polarizable. blank Covalent bonds are partially ionic because of polarizability of atoms. blank Kr and Sr2- are isoelectronic.arrow_forward
- Which molecule or ion has 30 valence electrons? O oxalate anion, C2042- pyrylium cation, C5H5O* O diphosphorus pentaoxide, P2S5 acetate anion, C2H3O2arrow_forwardArrange the following bonds in order of least to most polar: C-N Br-CI Br-Br Br-F Na-CI > >arrow_forwardWhat is the molecular geometry of an oxygen atom that has 1 bond (to nitrogen) and 3 lone pairs using the VSEPR model and the bond angles in between? Thanks.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY