
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
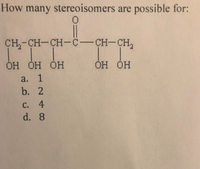
Transcribed Image Text:How many stereoisomers are possible for:
|
CH,-CH-CH-C-CH-CH,
ОН ОН ОН
ОН ОН
a. 1
b. 2
С. 4
d. 8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- 9L.9arrow_forwardThe anabolic steroid methandrostenolone is shown here. (a) How does it diff er structurally from testosterone? (b) Why might such drugs be administered to burn victims?arrow_forwardThe amino acid arginine ionizes according to the following scheme: NH, NH2 NH2 H NH, C=N C=N C=N C=N-H pk = 2.17 -H pK. = 8.99 -H pK = 125 -H* H. NH NH NH NH H (CH,)a H (CH), +H* (CH)a (CH,)a H,N-C-coo- +H* +H* H-N*-C-COOH H-N-C-Co- H,N-C-co- H. нн H. H II II IV (a) Calculate the isoelectric point of arginine. You can neglect contributions from form I. Why? (b) Calculate the average charge on arginine when pH = 9.20. (Hìnt: Find the average charge for each ionizable group and sum these together.) (c) Is the value of average charge you calculated in part b reasonable, given the pl you calculated in part a? Explain your answer.arrow_forward
- Which of the disaccharides shown in Figure 9-2 contain(s) two hexoses? Figure 9-2 CH2OH CH2OH OH H H OH H H OH OH H H OH H OH Disaccharide A CH2OH H HOCH2 H H OH H HO OH CH2OH H OH OH H Disaccharide B CH2OH CH2OH OH H H OH OH H H OH OH H OH Disaccharide Carrow_forward1.1 Which of the following statements is true for the chemical depicted below? CH,-CH,-CH,-CH,-CH2-CH,-CH,-CH,-CH2-CH2-CH,-CH3 CH3-N-CH3 CH,-CH,-CH,-CH,-CH2-CH,-CH,-CH,-CH,-CH,-CH,-CH3 A) it has unsaturated hydrocarbon chains B) this molecule is a cationic lipid c) this molecule readily dissolves in water D) this molecule could be useful for carrying nucleic acids into cellsarrow_forwardAn -OH group is a(n) _______ group.arrow_forward
- Select the choice that best describes the stereochemistry of the following amino acid, and rank the priority of the four groups surrounding the alpha carbon (in DESCENDING order: 1-highest 4-lowest) NH₂ CO₂II CH₂SH Cysteine H D and S, (1-NH2, 2-CH2SH, 3-COOH, 4-H) Land R, (1-NH2, 2-COOH, 3-CH2SH, 4-H) OL and R, (1-NH2, 2-CH2SH, 3-COOH, 4-H) D and S, (1-CH2SH, 2-COQH, 3-NH2, 4-H) Harrow_forwardCircle the functional groups on the R-group of the AMINO ACIDS below that are capable of forming hydrogen bonds. How do you know? 1 O H₂N-CH-C-O H 6 H₂N-CH-C-O CH H₂ CH₂ H₂C 2 요 H₂N-CH-C-O CH, 7 ar 3 hods word no 4hom on oq H₂N-CH-8-0-H₂N-CH-8-0 H₂N-CH-8-0- CH₂ CH₂ H₂C 201 8 CH₂ CH CH CH₂ sizoda H₂C CH₂ 10. Birds 9 H₂N-CH-8-0 H₂N-CH-8-0- CH₂ OH CH H₂C OH 578 aloriool H₂N-CH-C-O CH₂ CH₂ 0=C o 5 0=c NH₂ 요 H₂N-CH-C-O CH₂ CH₂ CH₂ CH ₂ 10 H₂N © University of California, Davisarrow_forwardDraw the enantiomer of the monosaccharide by changing the structure below. H ☑ H -ОН H -ОН но -H HO -H CH₂OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON