
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
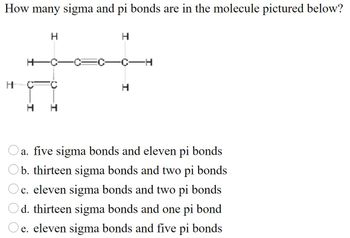
Transcribed Image Text:How many sigma and pi bonds are in the molecule pictured below?
H
H
H
a. five sigma bonds and eleven pi bonds
b. thirteen sigma bonds and two pi bonds
Oc. eleven sigma bonds and two pi bonds
Od. thirteen sigma bonds and one pi bond
e. eleven sigma bonds and five pi bonds
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- geNSUIortheastern St OWLv2 LOnline teaching. Chemistry Questions eA. vLAShort Paner: Alcohol/Foo. Centent O X Screenshot 2020-11-15 at 11.03.... O O Q Q ĮKererencesĮ a. Predict the molecular structure and bond angles for SeCle. Approximate bond angles are sufficient. Molecular Structure = Bond Angles b. Predict the molecular structure and bond angles for IC15. Approximate bond angles are sufficient. Molecular Structure = Bond Angles linear bent Try A 3 item attempts remaining ubmit Answer trigonal planar T-shaped trigonal pyramidal tetrahedral square pyramidal square planar octahedral 11:04arrow_forwardFor which of the following molecules will the electron-pair geometry be different than the molecular geometry? Draw Lewis structure for each molecule and write the electron pair and molecular geometries for each one in a separate sheet. a. BH3 b. H2O c. CH4 d. CO e. NH4+arrow_forwardWrite a Lewis structure for each of the following simple molecules. Show all bonding valenceelectron pairs as lines and all nonbonding valence electron pairs as dots.a. H2S b. SiF4 c. C2H4 d. C3H8arrow_forward
- Draw the Lewis structure for the molecule CH3CH2CCH. How many sigma and pi bonds does it contain? A. 8 sigma, 3 pi B. 9 sigma, 1 pi C. 11 sigma, 0 pi D. 8 sigma, 2 pi E. 9 sigma, 2 piarrow_forward6. Consider the ozone molecule, O₂. Draw the Lewis diagram for ozone. Include: i. A calculation for the number of valence electrons. ii. All resonance forms. iii. Name the molecular geometry of the molecule.arrow_forwardConsider the structure below. What is the electron geometry for the circled central atom? H H HH H. -CEC-H Harrow_forward
- 10arrow_forwardA. What is the electron-pair geometry for B in BF4-?fill in the blank 1 There are lone pair(s) around the central atom, so the geometry of BF4- is ________. B. What is the electron-pair geometry for Se in SeF6?fill in the blank 4 There are lone pair(s) around the central atom, so the geometry of SeF6 is _________.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY