
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
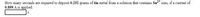
Transcribed Image Text:How many seconds are required to deposit 0.252 grams of tin metal from a solution that contains Sn* ions, if a current of
0.899 A is applied.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many grams of magnesium metal will be deposited from a solution that contains Mg2+ ions if a current of 0.724 A is applied for 39.4 minutes. gramsarrow_forwardHow many grams of magnesium metal will be deposited from a solution that contains Mg2+ ions if a current of 0.771 A is applied for 47.5 minutes. gramsarrow_forwardHow many seconds are required to deposit 0.223 grams of lead metal from a solution that contains Pb2+ ions, if a current of 0.772 A is applied.arrow_forward
- How many grams of cobalt metal will be deposited from a solution that contains Co2+ ions if a current of 0.974 A is applied for 67.4 minutes?arrow_forwardHow many grams of aluminum metal will be deposited from a solution that contains Al3+ ions if a current of 0.579 is applied for 29.5 minarrow_forwardHow many grams of tin metal will be deposited from a solution that contains Sn2+ ions if a current of 0.622 A is applied for 31.6 minutes.arrow_forward
- How many seconds are required to deposit 0.175 grams of cadmium metal from a solution that contains Cd2+ ions, if a current of 0.523 A is applied. sarrow_forwardHow many minutes would it take to deposit 5.71 g of Cu(s) from a solution of Cu(NO3)2 when using a current of 37.7 A?arrow_forwardHow many grams of iron metal will be deposited from a solution that contains Fe ions if a current of 0.640 A is applied for 32 minutes. grams 10:54 PM Bainarrow_forward
- 4arrow_forwardHow many seconds are required to deposit 0.278 grams of manganese metal from a solution that contains Mn2+ ions, if a current of 0.908 A is applied. Sarrow_forwardHow many grams of zinc metal will be deposited from a solution that contains Zn^2+ ions if a current of 1.08 A is applied for 55.8 minutes.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY