
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:b
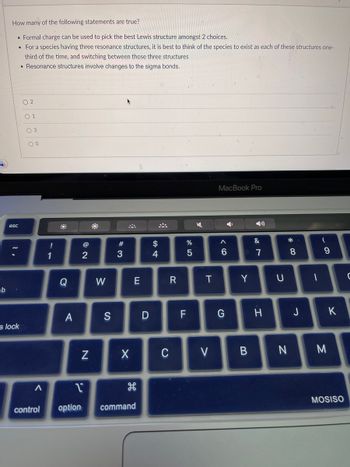
How many of the following statements are true?
• Formal charge can be used to pick the best Lewis structure amongst 2 choices.
• For a species having three resonance structures, it is best to think of the species to exist as each of these structures one-
third of the time, and switching between those three structures
Resonance structures involve changes to the sigma bonds.
esc
s lock
02
01
0 3
00
A
control
!
1
Q
A
2
N
W
S
#3
X
E
مه
1
option command
26
D
$
4
*
R
C
LL
F
%
5
K
T
V
MacBook Pro
Y
A
6
G
Y
&
7
H
U
*00
B N
8
J
-
(
9
M
K
MOSISO
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the Lewis structure for BrNO2 (N is central). Show resonance structures if applicable.arrow_forwardWhat are the best steps to follow when drawing a lewis structure? I'm getting conflicting steps when determining the central atom.Do you determine it based on electronegativity or if it's the element there's only one of?arrow_forwardAn incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of zero. H Н—N—С- -о—с—Н H H Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges. Click and drag to start drawing a structure.arrow_forward
- Decide whether this proposed Lewis structure is reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. : Z: : 0: N O C1: The correct number is: ☐ - No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". Xarrow_forwardHow many covalent bonds and how many ionic bonds are there in the molecular structures below? Please note that a pair of cation (+) and anion (-) form ONE ionic bond, and that a double bond counts for TWO covalent bonds.arrow_forwarded Lewis structure H-H-0 : Cl H10: Η HO H :0: U: :: + C-Cl: Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: * Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: * Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: *arrow_forward
- What are the formal charges of the resonance structure :C=S:=S::arrow_forwardWrite the Lewis structure for each molecule/ion. NO2-arrow_forwardCOMPARING LEWIS STRUCTURES TO2/S02 | =N = :o: Enter the overall charge for each substance. For a non-zero charge greater than one, enter the number first and then sign. Calculate the overall charge by summing the formal charge on each atom in the species. :ö:arrow_forward
- Writing Lewis Structures Many Lewis structures can be drawn by inspection but a system helps to draw the more complex ones. One approach is too : 1. Draw a skeleton of the major (non-hydrogen) atoms with single bonds ( lines) between each one. Several general considerations will help produce a valid skeletal diagram. normal covalency number or number of bonds that each atom forms. Consider the For the major atoms these are : Element Bond(s) HYDROGEN 1 BORON 3 ALUMINUM 3 CARBON | 4 NITROGEN 3 OXYGEN 2 HALOGENS 1 Also note that hydrogen, boron, aluminum and the halogens seldom form multiple bond.arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure :: : Cl - : 0: :0: :Z: C. C= : Z: N N Cl: Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* 0 Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". X 5arrow_forwardDetermine the formal charge for the atom indicated in the structures below. ASSUME that the structures are drawn correctly and that you do not have to change them due to formal charge issues. The external atom in this structure. :O: :O! Ar 11 :0: The central atom in this structure. = :0: (1 Si = : 0: 2- 1. +1 2. +2 3. +3 4. 0 5. -3 6.-2 7. -1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY