
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
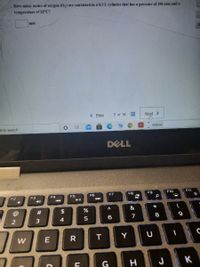
Transcribed Image Text:How many moles of oxygen (0,) are contained in a 8.3 L cylinder that has a pressure of 190 atm and a
temperature of 32°C?
mol
< Prev
7 of 14
Next >
Address
re to search
DELL
F7
F8
F9
F10
F11
F2
F3
F4
F5
F6
@
23
2$
7
3.
4
W
E
R
T
Y
U
GH
K
LL
Expert Solution
arrow_forward
Step 1
Given : Pressure of O2 = 190 atm
Volume of cylinder = 8.3 L
And temperature = 32 oC
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following containers are filled with nitrogen gas. Container 1: Volume = 2.0 L and Temperature = 15 C Container 2: Volume = 2.0 L and Temperature = 35 C Container 3: Volume = 5.0 L and Temperature = 15 C Container 4: Volume = 5.0 L and Temperature = 35 C Each container has the same pressure. Which one contains the highest number of gas moles?arrow_forwardA sample of hydrogen gas (H2) has a volume of 2.5 L at a temperature of 25°C and a pressure of 2.0 atm. Calculate the moles of H2 molecules present in this gas sample.arrow_forwardWhat pressure (atm) is needed to confine an ideal gas to 167.0 L after it has expanded from 35.0 L and 1.6 atm at constant temperature?arrow_forward
- A sample of hydrogen gas at a pressure of 1.18 atm and a temperature of 267 °C, occupies a volume of 612 mL. If the gas is heated at constant pressure until its volume is 737 mL, the temperature of the gas sample will be ____°C. (Explain well with proper step by step Answer. ).arrow_forwardA 21 ºC sample of oxygen gas has a pressure of 760 mm Hg. If the pressure is decreased to 0.958 atm, what is the new temperature of the gas?arrow_forwardA mixture of 10.0 g of Ne and 10.0 g Ar have a total pressure of 3.20 atm. What is the partial pressure of Ar?arrow_forward
- A mixture of hydrogen and xenon gases, in a 6.78 L flask at 18 °C, contains 0.426 grams of hydrogen and 21.5 grams of xenon. The partial pressure of xenon in the flask is__ atm and the total pressure in the flask is__ atm.arrow_forwardTrue or False? Consider 1 mole of gas A in a rigid 1.0 L container. If 1 mole of gas B is added to the same container, the pressure of each gas will double.arrow_forward4. Two containers are attached together as shown with a valve between the containers. The 26.0 L flask contains gas at a pressure of 4.25 atmospheres. The 47.0 L container does not contain any gas. The value between the two containers is opened and the gas in the 26.0 L container then also fills the 47.0 L container. What is the resulting pressure of the gas in the two containers?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY