
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
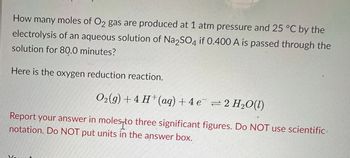
Transcribed Image Text:How many moles of O2 gas are produced at 1 atm pressure and 25 °C by the
electrolysis of an aqueous solution of Na2SO4 if 0.400 A is passed through the
solution for 80.0 minutes?
Here is the oxygen reduction reaction.
Oz(g)+4 H*(aq) +4e =2H,O(0)
Report your answer in moles to three significant figures. Do NOT use scientific
notation. Do NOT put units in the answer box.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An acidic solution of permanganate (MnO4–) ions can oxidize nitrous acid to nitrate ions. The products include dissolved Mn2+ ions. Write the chemical equations describing the oxidation of nitrous acid and the reduction of permanganate, and combine the two half-reactions to write a net ionic equation describing the overall redox reaction. Use the lowest whole-number coefficients possible and include states of matter. Define Oxidationarrow_forwardConsider the following equation in aqueous solution: PO₃³⁻(aq) + SO₂(g) → PO₄³⁻(aq) + S²⁻(aq) The oxidation half-reaction is PO₃³⁻(aq) + H₂O(l) → PO₄³⁻(aq) + 2 H⁺(aq) + 2 e⁻ and the reduction half-reaction is SO₂ (g) + 4 H⁺(aq) + 6 e⁻ → S²⁻ (aq) + 2 H₂O(l). How many moles of electrons must be transferred in the overall reaction?arrow_forwardNn.112. Subject:- Chemistryarrow_forward
- Be sure to answer all parts. Over a period of time, the concentration of sulfuric acid in the lead storage battery of an automobile has decreased from 38.0 percent by mass (density = 1.29 g/mL) to 26.0 percent by mass (1.19 g/mL). Assume the volume of the acid remains constant at 709 mL. (a) Calculate the total charge in coulombs supplied by the battery. x 10 с Enter your answer in scientific notation. (b) How long (in hours) will it take to recharge the battery back to the original sulfuric acid using a current of 23.4 A? harrow_forwardConsider the redox reaction shown below: IO3-1(aq) + 5 I-1 (aq) + 6 H+1 (aq) → 3 H2O (l) + 3 I2 (aq) What is the reducing agent?arrow_forwardThe amount of lactic acid, HC3H5O3, produced in a sample of muscle tissue was analyzed by reaction with hydroxide ion. Hydroxide ion was produced in the sample mixture by electrolysis. The cathode reaction is given below. 2 H2O(l) + 2 e− → H2(g) + 2 OH− (aq) Hydroxide ion reacts with lactic acid as soon as it is produced. The endpoint of the reaction is detected with an acid-base indicator. It required 104 s for a current of 13.1 mA to reach the endpoint. How many grams of lactic acid (a monoprotic acid) were present in the sample?arrow_forward
- Balance the following equation and write the sum of the coefficients of the reactants and products under basic conditions. BH41 is a hydride. BH4 0) + H2Oe → H3BO3laa) + H2a9) C 14 С 13 С 12 С 17 O 10 С 16 C 11 C 9 C 15arrow_forwardThe hydroperoxide ion, HO2–(aq), reacts with permanganate ion, MnO4–(aq) to produce MnO2(s) and oxygen gas. Balance the equation for the oxidation of hydroperoxide ion to O2(g) by permanganate ion in a basic solution.arrow_forwardSuppose 190. mmol of electrons must be transported from one side of an electrochemical cell to another in 21.0 minutes. Calculate the size electric current that must flow. Be sure your answer has the correct unit symbol and the correct number of significant digits. 0 x10 • X μ Śarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY