
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
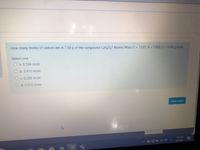
Transcribed Image Text:How many moles of carbon are in 7.50 g of the compound C3H4O2? Atomic Mass: C = 12.01; H = 1.008; O = 16.00 g/mole
Select one:
O a. 0.104 mole
O b. 0.416 mole
O c.0.208 mole
O d. 0.312 mole
Next page
7:05 PM
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2.50 moles of CH4 molecules contain ___ moles of H atoms. 1.51 x 10^24 0.625 5.00 10.0 2.50arrow_forwardIbuprofen is an anti-inflammatory with the formula C13H18O2 A. What is the molar mass of ibuprofen? Express your answer using four significant figures. B. How many grams of ibuprofen are in 0.505 mole? Express your answer using three significant figures. C. How many moles of carbon are in 12.5 g of ibuprofen? Express your answer using three significant figures. D. How many moles of ibuprofen contain 1.42×1023 atoms of carbon? Express your answer using three significant figures. please solve all stepwisearrow_forwardDetermine the empirical formula of a compound having the following composition: B a = 80.1%, 0 = 18.7%, H = 1.20%. B a O H Mass of element (in g) (assuming 100 g of compound) 1._80.1_ 2._18.7__ 3._1.20_ Moles of element 4.______ 5.______ 6.______ Moles of element/Smallest moles 7.______ 8.______ 9.______ Multiplier 10.______ 10.______ 10.______ Empirical formula Ba(OH)2arrow_forward
- he Tragedy of Ha... O 24 mol O 2.0 mol Carbon dioxide can be produced by burning carbon in oxygen: C + O₂ → CO₂ Approximately how many moles of carbon dioxide can be created from 24.0 g C? O 12 mol 22 mol O 44 mol O 0.20 mol O 0.50 mol 4.01 Content - Che... Q Search PRO CON Should Pit Bulls Be... CELOarrow_forwardO Emmaree Hernandez - Mole R X How to Calculate Molar Mass O Unit 4 Test Choice Boarc X A IN CLASS ASSIGNMENT FOR 1 X A goformative.com/formatives/6021509f9fbcc1919474d934 4 7. 8 9 10 5.717 grams H2 9 In the reaction: N, + 3H, 2NH, what is the mole ratio of hydrogen to nitrogen? 3. O3:2 1:1 1:2 О 3:1arrow_forward8. Calculate the mass in grams of 2.08 moles of (NH4)2SO4 9. Calculate the number of moles in 3.52 g of C,H%O6 ertrn Amabr swoliol s o dons niAarrow_forward
- + || 00 B # 3 Apps M Gmail YouTube Maps How many grams are in 0.500 moles of sodium bicarbonate? O 42.0 g O 42.0035 g O 0.00595 g MacBook Air 888 F4 DD F7 F2 F3 F5 F8 F10 F12 63 & $ 2 4. { [ R } H K. > option command command optionarrow_forwardHow many moles of C4F8 contain 4.95 \times 1024 fluorine atoms? 9.73 moles C4F 8 3.09 moles C4F8 1.03 moles C4F8 6.58 moles C4F8 8.22 moles C4F8arrow_forwardConsider the balanced chemical reaction below. What is the maximum number of moles of CO2 that can be produced if 45.2 mol of C2H4O2 and 76.5 mol of O2 react? C2H.O2(g) + 2 O:(g) → 2 CO:(g) + 2 H2O(g) 1 2 NEXT > Based on your knowledge of stoichiometry, set up the table below to determine the amounts of each reactant and product after the reaction goes to completion. C:H.O:(g) 2 O:(g) 2 CO:(g) 2 H:O(g) + + Before (mol) Change (mol) After (mol) 5 RESET 45.2 -45.2 76.5 -76.5 22.6 -22.6 90.4 -90.4 38.3 -38.3 153 -153 31.3 -31.3 62.6 -62.6 6.9 -6.9arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY