
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
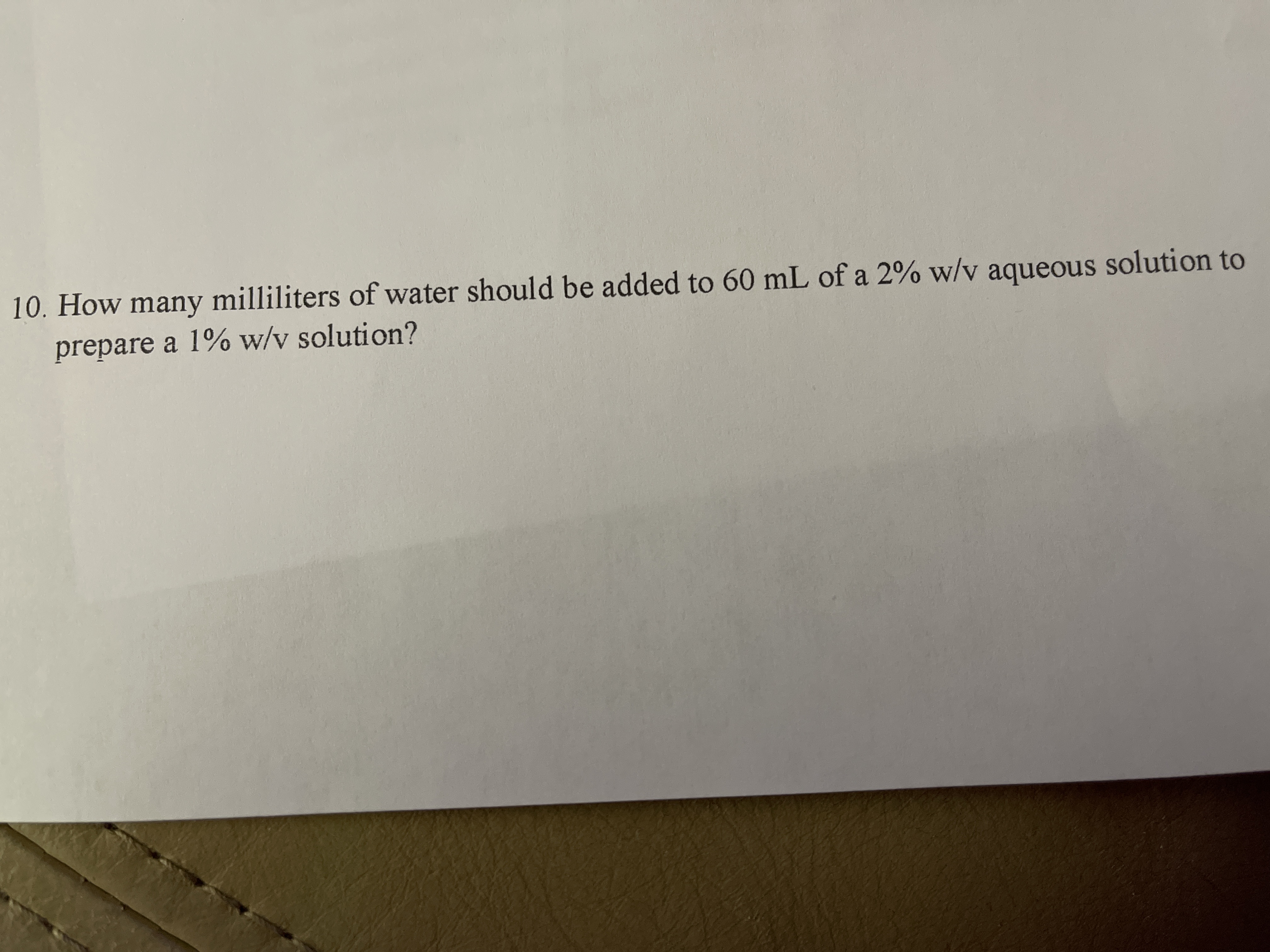
Transcribed Image Text:10. How many milliliters of water should be added to 60 mL of a 2% w/v aqueous solution to
prepare a 1% w/v solution?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How much NaCI (s) do you need to prepare 435 mL of a 2.5 % (m/v) NaCl solution?arrow_forwardHow many grams of sodium carbonate are needed to make 350 mL of a 0.5% (w/v)% concentrationarrow_forward1. How many milliliters of CHCl3 are neededto prepare a 2.5% v/v solution in 500 mL of methanol? 2. I a 250-mL solution of ethanol in water is prepared with 4mL of absolute ethanol, what will be the concentration of ethanol in % v/v?arrow_forward
- Answer these following questions please A) T.S.P. is an all purpose cleaner that can be used to clean driveways. What volume of solution would you get if you dissolved 150.0 g of sodium phosphate with water to produce a 0.23 mol/L solution? B) A "sports drink" contains 50 mg of sodium ions and 55 mg of potassium ions per 400 mL serving. Calculate the concentration of the sodium and potassium ions in ppm. C) What concentration results from mixing 400 mL of a 2.0 mol/L HNO3 solution with 600 mL of a 3.0 mol/L HNO3 solution?arrow_forwardHow many mL of H20 must be added to 25.0 of NAcl (solute) to prepare a 15% (v/v) NAcl solution?arrow_forwardCh 8 E #12 How many grams of water must be added to 48.2 g of NaOH in order to prepare a 7.42%(m/m) solution? grams of waterarrow_forward
- If a solution contains 128 Ml of ethanol and water to make 0.452L of solution what is the (v/v)% concentration?arrow_forwardHow many grams of NaCl would be needed to make a 500mL 1.5M NaCl solutionarrow_forwardPrepare 50 mL of an aqueous solution of 2.5% (w/v) NaCl (sodium chloride, salt).arrow_forward
- 1. What is the boiling point of a 3.62m solution of C2H6O2? 2. What is the melting point of a 3.6m C2H6O2?arrow_forwardWhen diluting a solution, what happens to the amount of solute and the amount of solvent in the solution? solute stays the same, solvent increases O solute increases, solvent stays the same solute stays the same, solvent stays the same solute decreases, solvent increases a 99+ bp 立arrow_forwardHow many grams of sodium chloride are contained in 300 ml of an 8% w/v solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY