
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
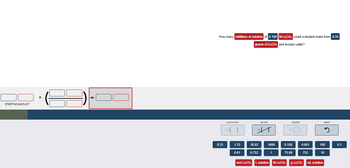
Transcribed Image Text:STARTING AMOUNT
X
How many milliliters of solution of 0.150 M LE-COs could a student make from 8.33
grams of Li:CO, and excess water?
8.33
ADD FACTOR
x( )
1.33
0.01
18.02
0.752
DELETE
A
1000
1
ANSWER
0.150
73.89
0.001
752
RESET
5
100
10
mol Li:CO: L solution M Li:CO: g Li:CO: mL solution
0.1
Expert Solution
arrow_forward
Step 1
Given =
concentration = 0.150 M
Wt . In gm = 8.33 g
Molecular wt = 73.89 =74 g/mol
73.88 g of Li2Co3 (lithium carbonate ) when dissolved in 1L of water we will get 1M solution .
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the number of grams of unknown solute (MW = 56.105 g/mol) in 250.0 mL of a 0.169 M solution. A) 2.37 B) 83.0 C) 0.753 D) 2.37 × 103arrow_forward8 pleasearrow_forwardCalculate the molarity of the solution when 1005 mL of solution contains 0.037 g of barium acetate. Is a molar mass needed for this calculation? Enter either yes or no. If so, calculate the molar mass using our periodic table and enter with the proper s.f. If not, enter 0. Do not include units. g/mol In the three blanks, enter the results of the calculation: value (decimal notation with proper s.f.), units, and substance (in this order).arrow_forward
- 100.0 mL of a 0.500 M solution of KBr is diluted to 500.0 mL. What is the new concentration of the solution? A) 2.50 M B) 0.500 M C) 0.100 M D) 0.0250 Marrow_forwardConvert the concentration of 0.700 M N22SO. to g/mL STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 119.05 83.3 0.001 99.4 1000 1 142.04 0.700 0.0994 0.0833 6.022 x 1023 g NazSO4 mol NazSO4 g/mL mL L M NazSO, g Na+ mol Natarrow_forwardcan you help me and line it exactly like this because i keep doing it and its says its wrongarrow_forward
- How can we find the volume for both questions?arrow_forwardPart of a buret measuring in mL is shown below. Read the volume to the proper number of significant digits. 45 46 Volume mL 47 48 49 2. Suppose it takes 33.69 mL of 0.005123 M EDTA to titrate 25.00 mL of a hard water solution. a) How many moles of EDTA were required to titrate to the endpoint? b) How many moles of hard water ions, expressed as moles of Ca?+, are present in the hard water solution? c) What is the concentration, expressed as molarity of Ca2+, of hard water ions in the solution? d) If the metal ions in the hard water all came from CaCO3, how many grams of CaCO3 would be in 1.000 L of the solution (in other words, determine g CaCO3/L)? e) Convert g CaCO3/L calculated in (d) to mg CaCO3/L. (This is equivalent to ppm.)arrow_forwardMISSED THIS? Watch KCV: Solution Concentration, IWE: Calculating Solution Concentration; Read Sections 5.2 and 5.4. You can click on the Review link to access the section in your e Text. What is the molarity of Br in each solution? Part A 0.500 M KBr Express your answer with the appropriate units. [Br] = Value Submit Part B μA Submit Request Answer 0.270 M CaBr2 Express your answer with the appropriate units. Part C [Br] = Value μА Request Answer O 0.500 M AlBr3 Express your answer with the appropriate units. μA Units [Br] =| Value Submit Request Answer Units Units P Pearson ? ? ?arrow_forward
- 4) did i do this right?arrow_forwardWhat NaCl concentration results when 239 mL of a 0.840 M NaCl solution is mixed with 582 mL of a 0.390 M NaCl solution? concentration: Marrow_forwardHow many milliliters of 8.64 M hydrobromic acid solution should be used to prepare 5.00 L of 0.100 M HBr? ____mLarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY