
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
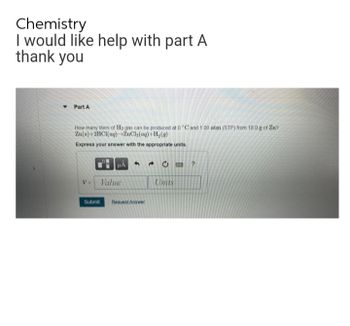
Transcribed Image Text:Chemistry
I would like help with part A
thank you
▾ Part A
How many liters of H₂ gas can be produced at 0 °C and 100 atm (STP) from 18.0 g of Zn?
Zn(s)+2HCl(aq) ZnCl₂(aq) +H₂(g)
Express your answer with the appropriate units.
HÅ
V Value
Submit Request Answer
0
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In order to produce solid iron, it first must bc processed from its orc, Fe2O1, in a blast furnace. During operation, CO gas (flue gas) is introduced into the furnace at 1650 °C and a pressure of 250 kPa. In this process flue gas reduces iron to its elemental form in the following reaction: 41. Fe;O30) COg Fe+ COz At one point in this process 256.0 L of CO was used to reduce iron ore. Assuming a complete reaction, how many grams of solid iron can be produced. Balanced chemical equation: Mass of Fe (in grams)arrow_forwardA mixture of He and CO2 is placed in a 7.5L flask at 22C. The partial pressure of the He is 2.2 atm and the partial pressure of the CO2 is 1.9 atm. What is the mole fraction of He?arrow_forward1) Calculate the volume of 1.62g of Helium gas at 25.670C and 872 mmHg. (R = 0.0821 atm.L/mol.K) 2) Given the following reaction of Ca(s) in HCl(aq): Ca(s) + 2 HCl(aq) --> CaCl2(aq) + H2(g) If 32.7g of calcium solid are placed in this reaction and at the end of the experiment, hydrogen gas is produced at 25.000C and with a pressure of 790. mmHg. Calculate the volume of hydrogen gas produced. 3) An unknown gas with a mass of 1.211g occupies a volume of 677 mL. The temperature is 230C and the air pressure is 0.987 atm. Calculate the molar mass of the gas.arrow_forward
- Ammonia, NH3, can react with oxygen to form nitrogen gas and water. 4 NH3(aq) + 30₂(g) →→→ 2N₂(g) + 6H₂O(1) If 3.85 g NH3 reacts with 5.78 g O₂ and produces 0.950 L N₂, at 295 K and 1.01 bar, which reactant is limiting? NH,(aq) 0₂(g) Calculate the percent yield of the reaction. percent yield:arrow_forwardCHEM 1 Please solve correctly and give the complete solution.arrow_forward1. Many laboratory gases are sold in steel cylinders with a volume of 43.8 L. If there are x kilograms of argon inside a cylinder whose pressure is 4,592 kPa at 46°C, what the value of x? (1 atm = 101325 Pa) 2. A tank was filled with 18 g of oxygen (O2), 75 g of nitrogen (N2) and 4 g of carbon dioxide(CO2). At 25°C the pressure of the tank was 8.5 atm. If the partial pressure of CO2 in the tank is x atm, what is the value of x? 3. 13 g of an unknown gas in a tank with a volume of 5.53 L has a pressure of 3.83 atm at 28°C. If the molecular weight of the gas is x g/mol, what is the value of x? 4. Ammonia burns in air as follows:NH3(g) + O2(g) → N2(g) + H2O(g) (unbalanced)If x liters of N2(g) are formed by burning 9.8 L of ammonia and 9.8 L of oxygen with all species at the same temperature and pressure, what is the value of x? 5. Methane (CH4) at 48°C and 776 mmHg has a density x g/L. What is the value of x? 6. A 3.50 L sample of neon has a pressure of 1.03 atm at 17°C. If the sample has…arrow_forward
- A 7.00 L tank at 15.8 °C is filled with 5.07 g of sulfur tetrafluoride gas and 11.5 g of sulfur hexafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. mole fraction: x10 sulfur tetrafluoride partial pressure: || atm mole fraction: sulfur hexafluoride partial pressure: atm Total pressure in tank: atmarrow_forwardConsider the following situation: You have a 50 L tank of compressed air at 15 atm and 100 ºC. You then pass the compressed air through a "titanium getter" which contains fragments of hot titanium metal. The purpose of the "getter" is to remove oxygen gas from the sample as Ti reacts with oxygen to form solid TiO2 (known as titania). Once the purified air is passed through the getter is it cooled back down to 100 ºC and fills another 50 L storage tank. If air typically contains about 20% oxygen, what could the pressure in the storage tank be? Question 2 options: A) 12 atm B) 15 atm C) 18 atm D) 24 atmarrow_forwardAn experiment is performed to determine the volume of gas produced by the following reaction: 2HCl(aq) + Mg(s) MgCl2(aq) + H2(g) A. What is the maximum mass of Mg in g that could be reacted at 20oC temperature and 1.0atm, if the volume of the gas burette is 50mL? (Hint: Find moles of H2 using the ideal gas law; then, convert to g of Mg.) B. If the experiment were moved to a higher elevation and the pressure was decreased to 0.95 atm would the maximum amount of Mg increase or decrease? C. If 16mg of Mg were reacted, what is the volume of gas produced in this reaction? D. What is the solvent for this reaction? E. Would the HCl(aq) be considered a strong, weak, or non-electrolyte? F. Would the MgCl2(aq) be considered a strong, weak, or non-electrolyte?arrow_forward
- 11. (a) In a 20.0 L steel container, we have only 77.7 g of CO2(g), 66.6 g of N2(g), and O2(g). The temperature is 25.0 ◦C and the total pressure is 8.88 atm. What mass of O2(g) do we have, and what is its partial pressure? The molar masses of C, N, and O are 12.01, 14.01, and 16.00 g/mol. (b) The density of a sample of pure CH4(g) at a constant pressure of 1.00 atm is 0.666 g/L. What is the average speed, or root mean square speed, of the CH4(g) molecules in this sample? The molar masses of C and H are 12.01 and 1.01 g/mol.arrow_forwardCalculating partial pressure in a gas mixture GHA 0/5 A 8.00 L tank at 24.9 °C is filled with 9.56 g of boron trifluoride gas and 4.99 g of sulfur tetrafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. mole fraction: boron trifluoride partial pressure: atm mole fraction: sulfur tetrafluoride partial pressure: atm Total pressure in tank: atm Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cen 42°F Cloudy ENC DELLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY