
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
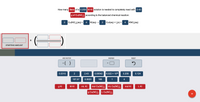
Transcribed Image Text:How many liters of a 0.209M KI solution is needed to completely react with 2.43
g of Cu(NO,), according to the balanced chemical reaction:
2 Cu(NO,),(aq) + 4
KI(aq) →
Cul(aq) + 1,(s) +
4 KNO,(aq)
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
0.0310
2.43
0.00542
6.022 x 1023
0.209
0.124
187.57
0.0620
166
1
4
g KI
M KI
mL KI
mol Cu(NO,), mL Cu(NO,),
mol KI
L KI
g Cu(NO,), L Cu(NO,),
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the neutralization reaction 2 HNO, (aq)+Ba(OH), (aq) 2 H, O(1)+Ba(NO,),(aq) A 0.115 L sample of an unknown HNO, solution required 36.7 mL of 0.250 M Ba(OH), for complete neutralization. What is the concentration of the HNO, solution? concentration: M * TOOLS x10arrow_forwardThe balanced equation for the neutralization reaction of aqueous H, SO, with aqueous KOH is shown. H,SO, (aq) + 2 KOH(aq) 2 H,O() + K,SO,(aq) What volume of 0.150 M KOH is needed to react completely with 11.2 mL of 0.300 M H, SO, ? volume: mLarrow_forwardassroom.collegeboard.org Question 10 Ⓒ Submit 44% 10:47 (:D) My Response A chemist performs a gravimetric analysis. The chemist combines 1.00 L of 2.00 M AgNO3(aq) with 1.00 L of 4.00 M NaCl (aq) in an Erlenmeyer flask. Both the AgNO3(aq) solution and the NaCl(aq) solution are colorless. After the mixture has been stirred, a cloudy white substance is observed at the bottom of the flask. : ||| 18 f(x)arrow_forward
- 1) For each pair of reactants, will there be a reaction? Co(s) + HCl(aq) Cu(s) + HCl(aq) Al(s) + HCl(aq) K(s) + Fe(C₂H30₂) 2 (aq) Reaction No reaction Reaction No reaction V Activity Seriesarrow_forwardQuestion 8 of 9 Submit Write the balanced molecular chemical equation for the reaction in aqueous solution for lead(II) nitrate and sodium chloride. If no reaction occurs, simply write only NR. 3C,2- Reset 1 2 3 4 5 6. 7 8 9 + (s) (1) (g) (aq) N NR Na Pb S NO CI • x H2O Tap here or pull up for additional resourcesarrow_forwardClassify each of the possible reactions according to whether a precipitate will form or will not form. Precipitate forms Na₂CO3(aq) + CuCl₂(aq) → ? CrCl,(aq) + Li,CO,(aq) — ? Answer Bank Na3PO4 (aq) + CoCl₂(aq) → ? No reaction KI(aq) + NaCl(aq) →→→ ? KNO3(aq) + NaCl(aq) →arrow_forward
- Nonearrow_forwardThe concentration of a Cu* solution is determined by titrating it with a 0.1395 M solution of permanganate. The balanced net ionic equation for the reaction is shown below. MnO, (aq) + 5 Cu*(aq)+8 H3O*(aq) Mn2*(aq) + 5 Cu2*(aq)+12 H,0(1) In one experiment, 21.54 mL of the 0.1395 M MnO, solution is required to react completely with 40.00 mL of the Cu* solution. Calculate the concentration of the Cut solution.arrow_forwardWhat volume (in mL) of 0.150 M HCI would be required to completely react with 4.30 g of Al in the following chemical reaction? 2 Al(s) + 6 HCI(aq) → 2 AICI, (aq) + 3 H,(g) 1 4 7 +/- MacBook Pro 888 %23 $ % 3 4 7 1 Q W E R Fab K A F lock C V M control option command * Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY