
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
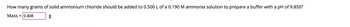
Transcribed Image Text:**Buffer Preparation Using Ammonium Chloride and Ammonia**
**Question:**
How many grams of solid ammonium chloride should be added to 0.500 L of a 0.190 M ammonia solution to prepare a buffer with a pH of 9.850?
**Answer:**
Mass = 0.936 g
---
**Explanation:**
To prepare a buffer solution with a desired pH, solid ammonium chloride is added to an ammonia solution. The precise amount required to reach a pH of 9.850 with the given volume and molarity of the ammonia solution is found to be 0.936 grams.
Understanding buffer solutions:
- Buffers are solutions that resist changes in pH when small amounts of acid or base are added.
- The ammonia (NH₃) acts as a weak base, while the ammonium chloride (NH₄Cl) provides the conjugate acid (NH₄⁺).
- This balance between NH₃ and NH₄⁺ maintains the pH close to the desired value, making it effective for buffering applications.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You need to prepare a pH = 9.20 buffer solution containing NH3 and NH4Cl. If you start with 0.71 M NH4Cl solution, what concentration of NH3 l will you need to also start with? (Kb for NH3 is 1.8x10-5)arrow_forwardThere are 0.250 ml of a Buffer solution, where the concentration is 0.225 M of sodium acetate and 0.225 M of acetic acid. What will the change in pH be if 30 ml of a 0.1 M HCl solution are added?arrow_forwardWhat [NH3] needs to be added to 0.0010M NH4Cl to obtain a buffer solution with pH of a 7.3?arrow_forward
- 3. A buffer solution contains 0.125 moles of ammonium chloride added to 500.0 ml of 0.500 M solution of ammonia. A) What is the pH of this buffer? B) What is the pH after the addition of 0.0100 moles of HCI bubbled into 500.0 mL of the buffer?arrow_forwardHow many moles of sodium hydroxide would have to be added to 225 mL of a 0.349 M nitrous acid solution, in order to prepare a buffer with a pH of 3.570? molesarrow_forwardHow many grams of dry NH4Cl need to be added to 2.50 L of a 0.300 M solution of ammonia, NH3 , to prepare a buffer solution that has a pH of 8.74? Kb for ammonia is 1.8×10−5 . (Please type answer no write by hend)arrow_forward
- 8b.arrow_forward7. A solution was made by mixing 50.0 mL of 0.750 M HCN with 25.0 mL of water. What is the pH of this solution? The pka of HCN = 9.31. 8. One of the compounds featured on the TV series "Breaking Bad" was hydrofluoric acid (HF), pKa = 3.14. a) A solution of HF has a pH = 1.77. What was the initial [HF] for this solution in mol L-1? b) What is the equilibrium concentration of F in this solution in mol L-1? c) What is the percent ionization of HF in this solution?arrow_forwardAn aqueous solution contains 0.386 M dimethylamine ((CH3)2NH).How many mL of 0.379 M perchloric acid would have to be added to 250 mL of this solution in order to prepare a buffer with a pH of 10.800.arrow_forward
- What is the pH of a buffer made by dissolving 0.2 mol of KBrO and 0.6 mol of HBrO in 850 mL of solution? What is the pH if the solution is diluted to 2 L?arrow_forward22. Which of the following will give a buffer solution when equal volumes of the two solutions are mixed? (a) 0.10 M HNO, and 0.10 M NaNO, ( b) 0.10 M HC,H,O, and 0.10 M Nac,H,O, (c) 0.10 M HC,H;O, and 0.20 M NaOH (d) 0.10 M HI and 0.20 M NaBrarrow_forward100. mL of 0.200 M HCl is titrated with 0.250 M NaOH. What is the pH of the solution after 50.0 mL of base has been added? What is the pH of the solution at the equivalence point?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY