
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
How many grams of NaNO3 will dissolve at 10°C?
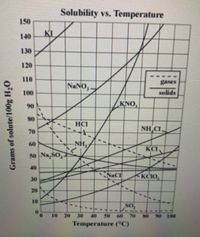
Transcribed Image Text:Solubility vs. Temperature
150
KI
140
130
120
110
gases
NANO,-
100
solids
90
KNO
80
HCI
NH CI.
70
60
-NH
KCI
50 Na, So,
40
NaC
KČIO,
30
20
10
10 20 30 40 50 60 70 80 90 100
Temperature (°C)
Grams of solute/100g H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- After Na2CO3 is added to the solution ___. Question 16 options: CaCO3 crystals form CO2 gas evolves Na2O precipitatesarrow_forwardWhat percent of a 0.0138 M NaCN solution undergoes hydrolysis? [K, for HCN-3.98E-10] 0.182 4.27 0.0000309 0.000234 4.57 6:51 PMarrow_forwardcalculate the number of mol of solute in 5.00 * 10^2 mL of 0.250 M HCLarrow_forward
- how many more grams of KNO3 will still dissolve in a solution of 20 grams of KNO3 at 20°C?arrow_forwardA solution at 25°C is 0.21 M KOH. What are the concentrations of H3O+ and OH− in this solution? (The value of Kw at 25°C is 1.0 10-14.) H3O+ M OH − Marrow_forward19.80 mL NaOH is required to titrate 0.6013 g of potassium hydrogen phthalate (KHC8H4O4). How many mL NaOH required to titrate 25.0 mL 0.10 M HCL solution?arrow_forward
- What is the concentration of A2+ after 30 drops of 0.400 M A2+ solution is added to 30 drops of water?arrow_forwardWhat mass of AgNO3 has been dissolved in 200g of H2O if the solution is 0.300m?arrow_forwardIn which glass material is 0.1 N HCl solution prepared? A-) beakerB-)balloon jojoC-)erlenmeyerD-)measureE-)nuche erleniarrow_forward
- What volume of 0.3M HNO3 will be required to react and neutralize 24ml of 0.25M KOH?arrow_forwardCalculate the pOH of a solution that is 0.165 M morphine C17H19O3N and 0.386 M morphine hydrochloride C17H20O3NCl. Kb of C17H19O3N is 1.6 x 10^-6arrow_forwardWhich of the units could be used to quantify the concentration of a solution of CUCI, ? V M.s-1 M mLarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY