
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How many grams of C a3(PO4)2 (MW = 310.18 g Ca3(PO4)2) are in 2.00E23 molecules of C a3(PO4)2?(Express as "g C a3(PO4)2")
2.00E23 molecules Ca3(PO4)2×1._2._=103 g Ca3(PO4)2
2.00E23 molecules C a 3 (P O4)2 times (1._ over 2._) = 103 g C a3 (P O4) 2
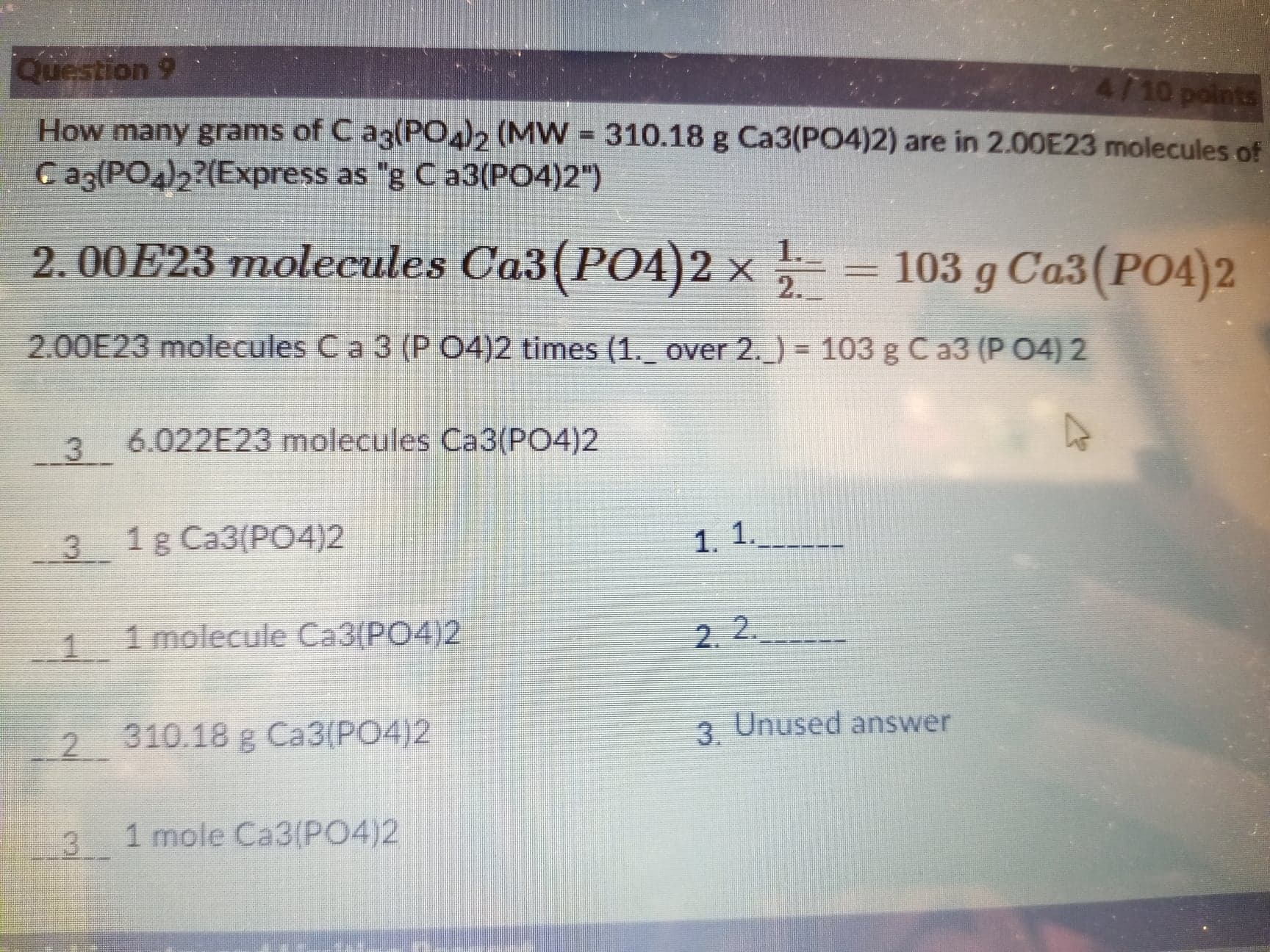
Transcribed Image Text:Question 9
4110points
How many grams of C az(PO), (MW = 310.18 g Ca3(PO4)2) are in 2.00E23 molecules of
C ag(PO4)2?(Express as "g C a3(PO4)2")
2. 00E23 molecules Ca3(PO4)2 x
1.
103 g Ca3 (PO4)2
2.00E23 molecules C a 3 (P 04)2 times (1. over 2. ) = 103 g Ca3 (P O4) 2
%3D
3 6.022E23 molecules Ca3(PO4)2
3 1g Ca3(P04)2
1. 1.
1 1 molecule Ca3(PO4)2
2. 2.
2.
310.18 g Ca3(PO4)2
3.
3 Unused answer
3
1 mole Ca3(P04)2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 10)arrow_forward2. A mixture of KCr(SO4)2 and its hydrate KCr(SO4)2-12H₂O has a mass of 1.2843 g. After heating to drive off all the water, the mass is only 1.1079 g. What is the weight percentage of KCr(SO4)2-12H₂O in the original mixture? Report your answer to 4 significant figures. Molar masses: KCr(SO4)2 283.22 g/mol; KCr(SO4)2-12H₂O 499.40 g/mol.arrow_forwardHow many moles of CH3OH are there in 86.2 mL of 0.400 М СН3ОН? |mol 1 3 6. C 7 8 +/- . + х 100 4-arrow_forward
- Copper metal reacts with nitric acid according the equation below 3 Cu (s) +8 HNO 3(aq) -...m+3 Cu^ 2+ (aq)+2NO (g) +6 NO 3 (aq)+4 H 2 O 0 What mass of NO (g) will be produced if 8.96 g of copper are reacted wtih excess nitric acid. Be sure to include units.arrow_forward4. How many moles of water are present in a 20.00 gram sample of potassium carbonate sesquihydrate, K2CO3 12 H2O? 5. How many grams of water would be lost if a 8.000 gram sample of PtCl4 5H20 were heated?arrow_forwardLiquid octane (CHa (LH2)6 CH.) will react with gaseous oxygen (0)) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). Suppose 10.3 g of octane is mixed with 55. g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction.arrow_forward
- d. Agt (6 pts) When an unknown hydrocarbon (Hy) is combusted and 6.309 g CO2 is produced. What is the empirical formula hol c. Na+ e. Cu²+ in excess oxygen, 3.444 g H₂O of the compound?arrow_forward2. A mixture of Na2SO4 and its hydrate Na2SO4-10H₂O has a mass of 1.5099 g. After heating to drive off all the water, the mass is only 1.1410 g. What is the weight percentage of Na2SO4.10H₂O in the original mixture? Report your answer to 4 significant figures. Molar masses: Na2SO4 142.04 g/mol; Na2SO4.10H₂O 322.19 g/mol.arrow_forwardHow many moles of Al are necessary to form 64.0 g of AIBR3 from this reaction: 2 Al(s) + 3 Br2(1) → 2 AIBR3(s) ? mol 4 5 6. 7 8 9. +/- х 100 3.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY