
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
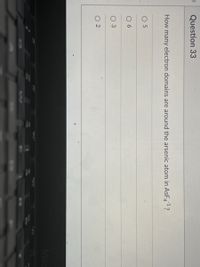
Transcribed Image Text:Question 33
How many electron domains are around the arsenic atom in ASF?
-1
O 5
O 6
O 2
Mac
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Let Su, Tn, and VI be imaginary elements in the same column in the periodic table. Their relative electronegativities are Su > Tn > VI and their relative atomic radii are VI > Tn > Su. Which of the following anions would have the highest K₁? ⒸHSUO₂ ⒸHVIO₂ ⒸHTnO₂ All of these would be expected to have the same Kb.arrow_forwardHow many double bonds are present in Yellow No. 6? (Include the S=O double bonds).arrow_forwardHow many valence electrons does ns^2 np^4 havearrow_forward
- Give the formula for the following molecule: trisilicon tetranitride Group of answer choices Zi3Ne4 Si3N4 S3N4 Si4N3arrow_forwardHow many electrons surround the sulfur atom in the SF molecule? 8 O 10 O 12 D<arrow_forwardUsing MO theory, which of the following would have unpaired electrons? O Li₂ O C₂ F₂ O N₂arrow_forward
- 5 identify the central atom(s) for HCCI3. CI H C * it is a check mark box type of questionarrow_forwardSharing ngs ols > The electronegativity values of representative elements in group 1A (1) to group 7A (17) 1 2 Group Group 1A 2A Li 1.0 Na 0.9 K 0.8 Rb 0.8 Cs 0.7 Be 1.5 Mg 1.2 Ca 1.0 Sr 1.0 Ba 0.9 H 2.1 13 14 15 16 17 Group Group Group Group Group 3A 4A 5A 6A 7A B 2.0 C 2.5 Al 1.5 1.8 Si P 2.1 ΤΙ 1.8 Ga Ge As 1.6 1.8 2.0 N 3.0 In Sn 1.7 Pb 1.9 O 3.5 Sb Te 1.8 1.9 2.1 Bi 1.9 S 2.5 Se 2.4 Po 2.0 F 4.0 Cl 3.0 Br 2.8 I 2.5 At 2.1 18 Group 8A Part A Show the dipole arrow for each of the following bonds. Drag the appropriate labels to their respective targets. 00 P-O S-CI P Pearson N-F Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining P-F Reset Help O-CI Torms of Use | Privacy Policy | Permissions | Contact Us |arrow_forwardWhich of the following is polar? CO2 SO2 CO32- SO3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY