
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
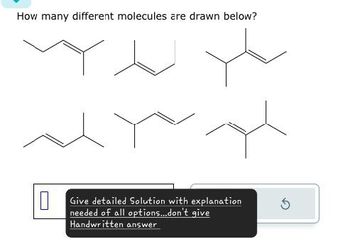
Transcribed Image Text:How many different molecules are drawn below?
☐
Give detailed Solution with explanation
needed of all options...don't give
Handwritten answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Solve and show all your work.arrow_forward6. If a Chemist has isolated 12 g PH3, How many molecules of PH3?arrow_forwardMake an individual table for each and every one of the following (7) molecules: 1. urea, (NH2) 2CO 2. ethyl thiomethyl ether, CH3SCH2CH3 3. ethyl chloride carbonate, CH3CH2OCOCl 4. trimethyl phosphite, (CH3O)3P 5. vinyl magnesium bromide, CH2CHMgBr 6. Cesium 2-hydroxyacetate, HOCH2CO2Cs 7. C4H8Br2 (an isomer).arrow_forward
- Nonearrow_forwardFormula of Calcium bicarbonate? ( including the positive ion and negative ion).arrow_forward19 10 15 6 7 18 11 12 13 14 15 16 17 Name brood Puzzled by the Concepts: 5 Naming 1 More Time Revised summer 2019-2022 S. Drake It is IMPORTANT to decide what kind of compound you have in order to name it. So, for the following compounds decide the type (simple ionic, transition metal ionic, polyatomic ionic, transition metal ionic & polyatomic ionic, or covalent) and then write its name or formula. Formula of compound 1 2 3 4 TIU2 Type of compound no (1) 1990 (V) muibansv hind aunorigaoriq byd munimuls ua (III) lexbin P Na₂CO3 P₂O5 CoBr2 FeSO4 SiO₂ CO SI CuCl2 SO₂ FeS NaBr Ca(C₂H302)2 Ti(SO4)2 FePO4 K3N CuOH Zn(NO₂)2 V₂S3 Tanel P CHE 106-17 Name of compound DO NOT capitalize. Disodium Carbonate T14arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY