
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
PLEASE MAKE SURE TO BOLD OR HIGHLIGHT WHAT BONDS!!
Also, highlight each bond in this structure that is trans but which could be cis in a different cis/trans isomer.
please and thank you
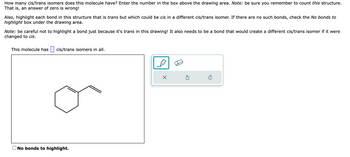
Transcribed Image Text:How many cis/trans isomers does this molecule have? Enter the number in the box above the drawing area. Note: be sure you remember to count this structure.
That is, an answer of zero is wrong!
Also, highlight each bond in this structure that is trans but which could be cis in a different cis/trans isomer. If there are no such bonds, check the No bonds to
highlight box under the drawing area.
Note: be careful not to highlight a bond just because it's trans in this drawing! It also needs to be a bond that would create a different cis/trans isomer if it were
changed to cis.
This molecule has cis/trans isomers in all.
No bonds to highlight.
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- PRE-LAB QUESTION After consulting sections 5.7 of your text, draw the complete MO diagram for the diatomic molecule, H2. Next to each of the MOs in your diagram, draw a picture that describes the constructive or destructive interference of atomic wave functions and indicate the location of any nodes. Using arrows to represent electrons, place one electron in each of the original atomic 1s orbitals at the edges of your diagram and then show how these electrons pair in the o1s bonding molecular orbital to form the bond that holds these two atoms together.arrow_forwardPlease don't provide handwriting solutionarrow_forwardDraw two constitutional isomers that share the molecular formula C₂H6O. Your structures will have the same molecular formula but will have different connectivities. 0 Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. +▾ *** ChemDoodleⓇ 00. [Farrow_forward
- How many cis/trans isomers does this molecule have? Enter the number in the box above the drawing area. Note: be sure you remember to count That is, an answer of zero is wrong! Also, highlight each bond in this structure that is trans but which could be cis in a different cis/trans isomer. If there are no such bonds, check the A highlight box under the drawing area. Note: be careful not to highlight a bond just because it's trans in this drawing! It also needs to be a bond that would create a different cis/trans isor changed to cis. This molecule has cis/trans isomers in all. u HO No bonds to highlight. OHarrow_forwardMN.49.arrow_forwardQuestion 14 A functional group capable of having characteristic electronic transitions. These chemical groups scatters light at a specific frequency and so imparts color to a molecule. Which of these statements is correct? O Only the first statement is correct O Only the second statement is correct O Both statements are correct O None of the statements is correctarrow_forward
- Please don't provide handwriting solutionarrow_forwardAlso, highlight each bond in this structure that is cis but which could be trans in a different cis/trans isomer. If there are no such bonds, check the No bonds to highlight box under the drawing area. Note: be careful not to highlight a bond just because it's cis in this drawing! It also needs to be a bond that would create a different cis/trans isomer if it were changed to trans. This molecule has cis/trans isomers in all. G Carrow_forwardHow many cis/trans isomers does this molecule have? Enter the number in the box above the drawing area. Note: be sure you remember to count this structure. That is, an answer of zero is wrong! Also, highlight each bond in this structure that is trans but which could be cis in a different cis/trans isomer. If there are no such bonds, check the No bonds to highlight box under the drawing area. Note: be careful not to highlight a bond just because it's trans in this drawing! It also needs to be a bond that would create a different cis/trans isomer if it were changed to cis. This molecule has ☐ cis/trans isomers in all. No bonds to highlight. Rarrow_forward
- K Please don't provide the handwriting solutionarrow_forwardUse the References to access important values if needed for this question. Including the cis or trans designation, what is the IUPAC name of the following substance? It is not necessary to put cis or trans in italics. Submit Answer Try Another Version 9 item attempts remainingarrow_forward. Draw a condensed structure for 2,3-dimethylbutane. Then draw condensed structures for the linear isomer and a branched isomer of that compound, and name those compounds 2,3-dimethylbutane Linear isomer ______________________________Name Branched Isomer ______________________________Namearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY