
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
show solution
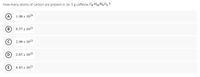
Transcribed Image Text:How many atoms of carbon are present in 34. 5 g caffeine, C3 H1,N402 ?
(A
1.08 x 1024
(в
8.57 x 1023
2.09 x 1023
(D
2.67 x 1025
E
4.83 x 1023
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- For each described change, determine the generally expected impact on a salt's solubility. Cooling a saturated solution by 10 °C Choose... Placing a saturated solution in a hot water bath Choose... Doubling the amount of salt added to a saturated solution Choose...arrow_forwardUsing a graduated flask (200mL or 250 mL) make volume up an appropriate an appropriate volume of an approximately 0.1M 101. Molard 0.1 moles of solute in 1 litre of solution.) 1. Calculate the mass of Navett needed. ofarrow_forwardUse the Henderson-Hasselbalch equation to calculate the pH of each solution:arrow_forward
- A. (see pic) B. When an electrolyte solution (such as NaCl) is added to ice, the ions have ____ with water molecules and thus ____ the ability of the water molecules to form a solid ice structure. C. Given that water's freezing pt is 0C and the fp depression constant is 1.86 Ckg/mol, calculate the fp depression for a 3.55 molal soln (moles/kg) of NaCl in water. Assume ideal behavior of the ions. D. Given that water's fp is 0C and fp depression constant is 1.86 Ckg/mol, calculate the fp depression for 3.55 molal solution for CaCl2 in water. Assume ideal behavior of the ions.arrow_forwardWith correct sig figsarrow_forwarda) b) as per guidelines you can answer these two question because they are bits of the same questionarrow_forward
- An aqueous BSA* solution in the tube on the left is in equilibrium with pure water in the tube on the right. The membrane that separates the two solutions is only permeable to water. When salt is added to the pure water on the right, aqueous BSA pure water H,0 permeable membrane the water level on the right increases c. d. no change in the levels occur none of the choices а. b. the BSA solution level on the left increases If salt is added to water, the fugacity of the water а. increases с. remains the same b. decreases d. none of the choicesarrow_forwardMatch each definition or prompt with the correct term on the right. The term used for a [Choose ] solution where water is the solvent. Water is considered this, due for its [Choose ] ✪ ubiquity in solutions. Water can conduct [Choose] ✪ an electric current with these dissolved in solution On it's own, pure water has a virtually [Choose ] ◆ no ability to do this to an electric current. In a saltwater solution, the salt is [Choose ] considered this. Glucose and other [Choose ] covalent compounds (except acids) are consider this when acting as solutes, and unable to carry current. î îarrow_forwardUsing the image attached please write 3 paragraphs 1st paragraph: objective stated clearly 2nd paragraph: complete condense method describe 3rd paragraph: analysis explained Please please please answer everything it’s very important please answer fastarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY