
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
How do I find t1/2 and concentration?

Transcribed Image Text:Given an initial cyclopropane concentration of 0.00810 M, calculate the concentration of cyclopropane that remains after
1.80 hours.
concentration:
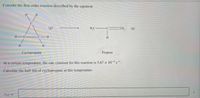
Transcribed Image Text:Consider the first-order reaction described by the equation
H
H
H,C-
cCH,
(g)
H.
H.
Cyclopropane
Propene
At a certain temperature, the rate constant for this reaction is 5.67 x 10-s-.
Calculate the half-life of cyclopropane at this temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- P = P2O5 × 0.44 P2O5 = P × 2.29 K = K2O × 0.83 K2O = K × 1.21 1 tonne = 1,000 kg 1 ha = 10,000 m2 Using the conversions above calculate : 1.How much N and K are in a 25 kg bag of 12-22-18? 2. How much P2O5 and P are in a 20 kg bag of 0-46-0? 3. How much K2O and P2O5 are in a 35 kg bag of 12-22-18?arrow_forwardGiven the Ksp = 1.4 x 10-13, what is the solubility of CoCO3 (i.e., what is the [Co2+] concentration) in water?arrow_forwardHow many grams of Ba(103)2 (487 g/mol) can be dissolved in 400 mL of water at 25°C. (Ksp of Ba(1O3)2 = 1.57 x 10-9) Ba(103)2 Ba2+ + 2103 O 0.298 g 0.122 g 0.221 g O 0.926 g O 0.143 garrow_forward
- Question 1arrow_forwardtab caps lock You have a 11.5 mg sample of blood that contains various proteins. Hemoglobin is the only protein in the sample containing Fe. Hemoglobin contains 3.83% by mass of a compound called heme (C34H32FeN4O4, MW = 616.49 g/mol). You find that your 11.5 mg blood sample contains 29.7 micrograms of Fe. What is the mass percent of hemoglobin in your protein sample? Tap here or pull up for additional resources Q A NO 2 W S #3 E D GA 4 R % 5 F Question 9 of 17 T 6 G Y & 7 B H 8 N 9 4 +/ V Marrow_forwardMode of separation that uses a polar stationary phase: IEX HILIC SEC NPLCarrow_forward
- The K₂ for HCN is 4.9 x 10-10. What is the value of Kb for CN? 2.0 × 10⁹ 2.0 x 10-5 4.0 x 10-6 4.9 x 104 4.9 × 10-24arrow_forward2. Run V (NH4)2S₂08 (mL) 20.00 20.00 10.00 5.00 20.00 15.00 1 2 3 4 5 6 [S₂082-] (mol/L) 0.04 0.04. 0.02 0.01 10.09 10.03. VKI (mL) 20.00 10.00 20.00 20.00 8.00 15.00 [1-] (mol/L) 0.08 0.07 0.08 0.08 0.032 V Na₂S₂03 (mL) 10.00 10.00 10.00 10.00 10.00 10.00 [S₂032-] (mol/L) 6.001 0.001 0.001 10.001 0.001 0.001 These calculated concentrations should be entered in the Data Table for this experiment for runs 1-6. To calculate the concentration of [12], use Equation (3) and the concentration of [S₂03²]. In a constant-temperature run described in the procedure section of this experiment, the solution turned blue in 50 s. a) Consider the concentration of [S₂032] in the mixed solution shown in the las column of the table from the previous exercise. Calculate the concentration c [12] final formed in this reaction. b) Calculate the rate of the reaction.arrow_forwarduのり19) WIWwb2 り1US1 2.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY