
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How do I classify all the statements ? Or convert them ?
Transcribed Image Text:Chapter 1, Problem 18P
W Bookmark
Show all steps:
ON
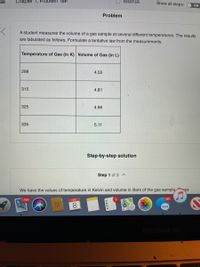
Problem
A student measures the volume of a gas sample at several different temperatures. The results
are tabulated as follows. Formulate a tentative law from the measurements.
Temperature of Gas (in K) Volume of Gas (in L)
298
4.55
315
4.81
325
4.96
335
5.11
Step-by-step solution
Step 1 of 3
We have the values of temperature in Kelvin and volume in liters of the gas sample ase
1,190
FEB
3
261
8.
30
MacBook Air
Expert Solution
arrow_forward
Step 1
According to Charles Law, the volume of a gas increases proportionally when the temperature in increased by keeping the pressure constant. Mathematically it can be shown as follows,
VT
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I do t understand the explanation given last tine on how to solve once .08/a = .012/b = .016c can this be better explainedThis is not a graded question as it is a practice question . I am 60 years old and helping my son prepare for the AP exam in a few months. We do questions at the back of the textbook by Zumdahl and Zumdahlarrow_forwarda white powder is cooled from the cruciblearrow_forward2nd - Which of the following is false for mixtures? a) Mixtures have the properties of the substances that make them up. B) Sandy water is an example of a heterogeneous mixture. NS) Cologne is an example of a homogeneous mixture. D) Mixtures are separated into their components by chemical method. TO) Mixtures are divided into homogeneous and heterogeneous.arrow_forward
- Write the balanced chemical equation for the preparation of carbon dioxide, given the following description: Acetic acid (vinegar, C2H4O2) is mixed with sodium bicarbonate (baking soda, NaHCO3) to form sodium acetate (NaC2H3O2), water, and carbon dioxide. Q6. Write the balanced chemical equation for the preparation of oxygen, given the following description: hydrogen peroxide (H2O2) reacts to form water and oxygen. Don't forget that oxygen gas is formed as a diatomic molecule!arrow_forward4b. Convert the mass of the graduated cylinder with water using an analytical balance from grams to tons. 98.5107 g 4 4 13.8 mL 13.8 mL X 4 X 4 5. Beginning with the volume of water measured by the graduated cylinder, convert the volume from milliliters to liters and microliters. 4 4 4 P 4 15 15 L (rounded) HL 14 tonsarrow_forwardEarth's oceans have an average depth of 3800 m, a total area of 3.63 x 10 km², and an average concentration of dissolved gold of 5.8 × 10. If a recent $987.00 troy oz what is the value of gold in the oceans? (I troy oz 31.1 g) Round your answer to 2 significant figures. Note: Reference the SI prefixes and Unit conversions for derived SI units tables for additional information. price of gold was 0arrow_forward
- Is the boiling point of a chemical a physical property or chemical property?arrow_forwardI'm not sure how to do this number 8arrow_forward2. Are the following chemical (C) or physical changes (P)? Sugar dissolves in water b) Sugar is heated to form caramel. Water evaporates. d) Charcoal burns on a grill.arrow_forward
- 1. Classify each of the following as a physical change or a chemical change. For each chemical change, explain how you can tell that a substance has been formed. a) Water boils then turns into steam. b) Wood sawed and made into a toy box. c) Firewood burns and ashes remain. d) Orange drink crystals are stirred into a pitcher of water. e) Sugar, eggs, and flour are mixed and baked into cookies.arrow_forwardCopper pennies Pour about 2 Tbs of vinegar into the bowl and add a 4 tsp of salt stir it up. Put about 5 pennies into the bowl and count the pennies to sit in the vinegar salt mixture for 5 minutes. Take out the pennies and rinse them out in some water. 1. What happens to the pennies? 2. Is it a physical or chemical change? Explain.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY