
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
How could we use the
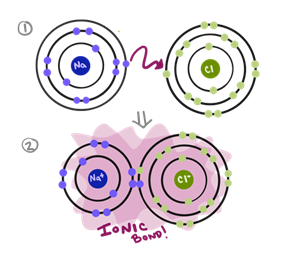
Transcribed Image Text:### Explaining Ionic Bonding with Sodium and Chlorine
**Diagram Description:**
This image illustrates the process of ionic bonding between sodium (Na) and chlorine (Cl) atoms.
1. **Initial State:**
- **Sodium (Na) Atom:**
- The sodium atom is represented with three concentric circles, indicating its electron shells.
- The inner and second shells are filled, and the outermost shell has one electron (depicted in blue).
- **Chlorine (Cl) Atom:**
- The chlorine atom is shown with three concentric circles.
- The inner two shells are filled, while the outermost shell has seven electrons (depicted in green).
- An arrow indicates the transfer of the outermost electron from sodium to chlorine.
2. **Ionic Bond Formation:**
- **Sodium Ion (Na⁺):**
- After losing its outer electron, sodium becomes positively charged (Na⁺) and has a full outer shell.
- **Chloride Ion (Cl⁻):**
- Chlorine gains an electron, resulting in a negatively charged ion (Cl⁻) with a complete outer shell.
- The purple background highlights the formation of an "ionic bond," where the oppositely charged ions attract each other, creating a stable compound.
This diagram effectively demonstrates how the transfer of electrons leads to the formation of ions and the ionic bond, a fundamental concept in chemistry.

Transcribed Image Text:### What Types of Elements Form Ionic Bonds and Why?
**Understanding Ionic Bonds:**
Ionic bonds are a type of chemical bond that occurs between metals and nonmetals. These bonds form through the complete transfer of valence electrons from one atom to another.
**The Role of the Octet Rule:**
The process of ionic bonding is driven by the Octet Rule, which suggests that atoms are most stable when they have eight electrons in their valence shell. As mentioned in the text:
- An atom can satisfy the Octet Rule by transferring valence electrons from one atom to another.
**Formation of Ionic Bonds:**
To achieve stability via the Octet Rule, a metal atom will donate its valence electrons to a nonmetal atom. Through this electron transfer, the metal becomes a positively charged ion, while the nonmetal becomes a negatively charged ion. The opposite charges attract, resulting in the formation of an ionic bond.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. (Short Essay) In the classical model of a Hydrogen atom, we can assume that an electron (e-) orbits about a nucleus containing the neutron and proton (e+). This is known has the Bohr Model of the hydrogen atom. Derive an expression for the total energy of an electron starting with Coulomb forces of an electron orbiting in a perfect circular motion. We can note that the potential energy between charges are just like with work, w = | Fglq2 · dr W Lets do some research now. With Bohr's model there were flaws. Why is this model incorrect? and what is the correct model of a Hydrogen atom? Provide a citation and works that you have looked into (websites/wikipedia are OK). There is no need to write more than one page (half is sufficient).arrow_forwardWhen we draw Bohr diagram for the elements, why calcium has 8 electrons on the third shell but zinc has 18 electrons on the Third shell. Justify your answer by writing the electron configuration and drawing Bohr diagram.arrow_forwardIs it possible to determine the position and energy of an electron at the same time? Why or why not?arrow_forward
- For the first box you are comparing Lithium and Sodium. Second box you are comparing Nitrogen and Oxygen. Last box you are comparing Sodium and Oxygen. Your answer to the questions should be based on where these elements are located on the periodic table. For example, “Strontium has a greater atomic radius because it is in period 5 and thus has 5 energy levels (while magnesium is in period 3 and only has 3 energy levels).”arrow_forwardHow are the Bohr model and the Rutherford model of the atom similar? How are they different?arrow_forwardI need to be able to know whether the elements in groups 1A and 2A gain or lose electrons to achieve noble gas configuration. I know that they lose electrons but I don't really understand why.arrow_forward
- ect mheducation.com y Chemistry: An Atoms First Approach-Burdge/Driessen, 2e, Electrons and the Periodic Table Type your answer in the box. An electron in a hydrogen atom whose energy is as low as it can possibly be is said to be in the state, whereas an electron with a higher energy is said to be In aln) excited state. Do you know the answer? Tknow it Think so Unsure No idea 71 ems len 99+arrow_forwardHow did the development of quantum mechanics change Bohr's model of the atom? a Instead of being spread evenly throughout the atom, the electrons were in a cloud around the outside. b Instead of being a broad cloud of electrons, the electrons were organized into discrete levels, or shells. c Instead of being in open shells, the electrons were placed into orbitals based upon their probability distribution. d Instead of being on the outside of the atom, the electrons were found to be in a separate space.arrow_forward2. Draw a Bohr model for the following atoms: a Neutral sulfur and sulfur ion. b. Magnesium-24 and magnesium-26 c. Neutral potassium and potassium ion.arrow_forward
- Is a Carbon Atom smaller than a silicon Atom?arrow_forward1. What are the 3 subatomic particles? Next to each give their location, charge and relative mass. 2. What does atomic number tell you about an atom? 3. What does mass number tell you about an atom? 4. What takes up the greatest amount of space in an atom? 5. Contrast the following models of the atom: Dalton's, Thompson's, Bohr's, and Quantum modelarrow_forward2, How are Bohr and Rutherford model of an atom similar? How are they different ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY