
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
How are wavelength and frequency related?
A. Wavelength is independent of frequency
B. Wavelength is one-half of the frequency
C. Wavelength increases as the frequency decreases
Expert Solution
arrow_forward
Step 1
Wavelength is the distance between the two point’s successive highest points or lowest points in a wave. It is measured in the direction of wave.
Frequency is the number of repetitions of one complete wave cycle.
arrow_forward
Step 2
In the electromagnetic spectrum there are many different types of waves with varying frequencies and wavelengths. They are all related by one important equation: Any electromagnetic wave's frequency multiplied by its wavelength equals the speed of light.
FREQUENCY x WAVELENGTH = SPEED OF LIGHT
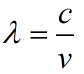
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ultraviolet (UV) radiation can be subdivided into three regions: UVA, UVB, and UVC, based on their energy. What are the minimum and maximum values of wavelength in the UVC region? Minimum wavelength (UVC): Maximum wavelength (UVC): nm nmarrow_forwardIf blue light has a wavelength of 400 nm and infrared light has a wavelength of 800 nm, what is the frequency of the blue light compared to the infrared?arrow_forwardWhat wavelength is associated with a green light that has a frequency of 6.01*10^14 Hz? A. 499nm B. 235 nm C. 165 nm D. 980 nmarrow_forward
- This is from a study guide for a final, not a graded assignment.arrow_forwardMicrowave radiation has wavelengths from 1.0×10-3 to 1.0 m, whereas the wavelength region for X-ray radiation is 1.0×10-11 to 1.0×10-8 m.We can say that: 1. The frequency of microwave radiation is X-ray radiation. 2. The speed of microwave radiation is X-ray radiation.arrow_forwardBecause the atoms of a given element emit only certain photons of light, only certain_____ occur in those particular atoms.arrow_forward
- An FM radio station broadcasts electromagnetic radiation at a frequency of 90.2 MHz. The wavelength of this radiation is A. 2.71 × 1010 B. 2.71 × 1016 C. 0.301 D. 3.33 E. 3.33 × 106arrow_forwardIR-Absorption is different from Atomic line spectra because a. a photon is absorbed but not emitted this frequency range b. a photon is emitted but not absorbed in this frequency range c. it affects electrons that are more closely held towards the atomic core of the atom d. it involves higher energy and shorter wavelengths e. the energy transitions are not related to the electron orbitalsarrow_forwardThe source of a wave is moving at 40 vibrations per second. What is the frequency of the wave it produces? A. 1 hz B. 40 hz C. 20 hz D. 80 hzarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY